Home /
Expert Answers /
Chemistry /
calculate-the-boiling-and-freezing-points-of-0-103mol-kg-aqueous-solutions-of-nacl-and-of-cacl2-pa851
Expert Answer
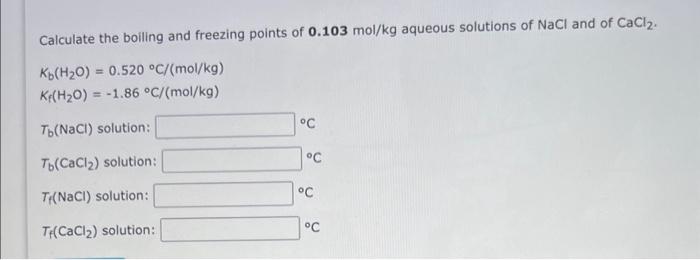
?Tb = i Kb xm NaCl = 2 x 0.52x 0.103m =0.10712 C