Home /
Expert Answers /
Chemistry /
calculate-the-activation-energy-for-the-reaction-understanding-the-high-temperature-behavior-of-nit-pa377
(Solved): Calculate the activation energy for the reaction. Understanding the high-temperature behavior of nit ...
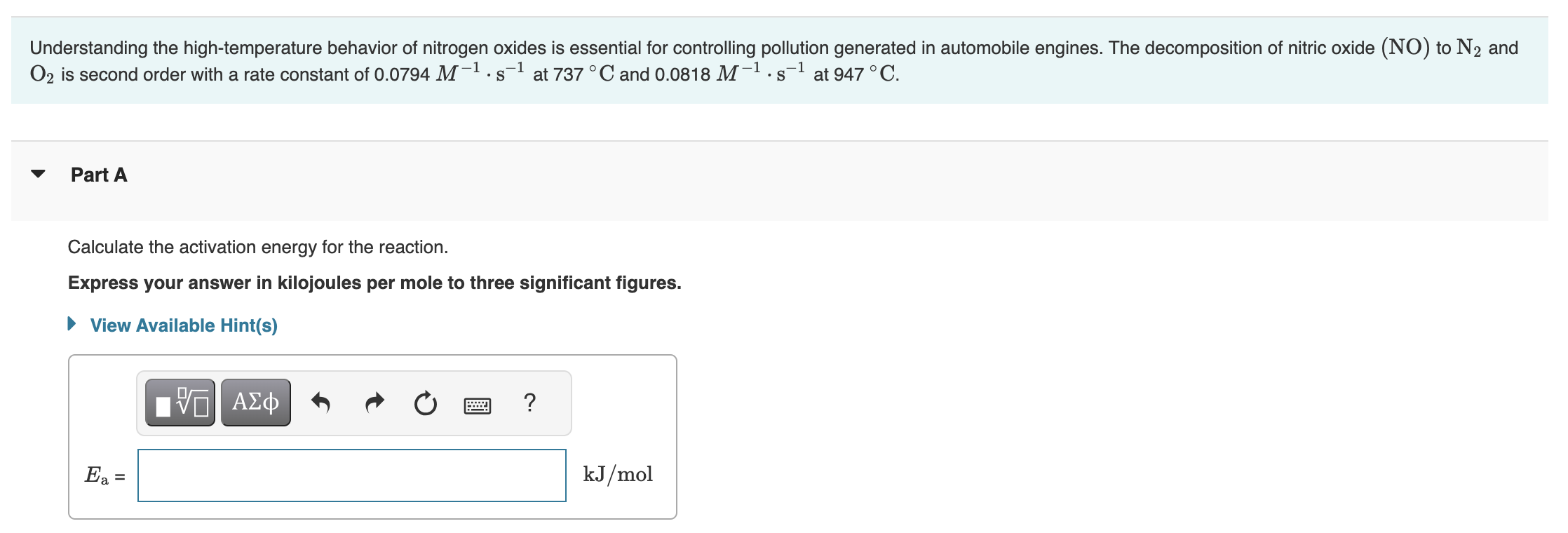
Calculate the activation energy for the reaction.
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollution generated in automobile engines. The decomposition of nitric oxide (NO) to and is second order with a rate constant of at and at . Part A Calculate the activation energy for the reaction. Express your answer in kilojoules per mole to three significant figures.