Home /
Expert Answers /
Chemistry /
calculate-overall-energy-changes-in-kilojoules-per-mole-for-the-formation-of-cacl-from-the-elements-pa792
(Solved): Calculate overall energy changes in kilojoules per mole for the formation of CaCl from the elements ...
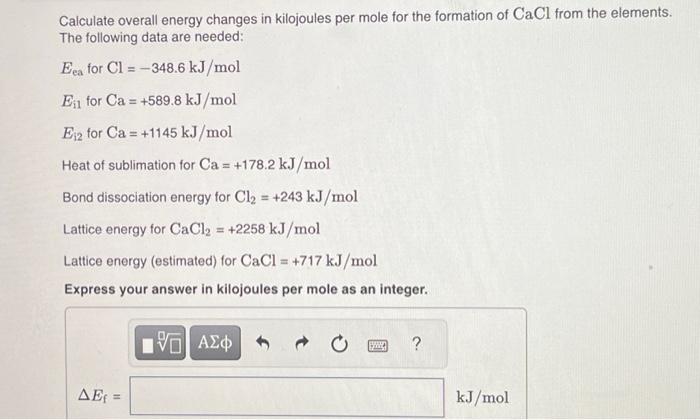
Calculate overall energy changes in kilojoules per mole for the formation of from the elements. The following data are needed: Heat of sublimation for Bond dissociation energy for Lattice energy for Lattice energy (estimated) for Express your answer in kilojoules per mole as an integer.