Home /
Expert Answers /
Chemistry /
calculate-g-in-kj-for-the-following-redox-reaction-under-standard-conditions-cu2-aq-pb-s-pa431
Expert Answer
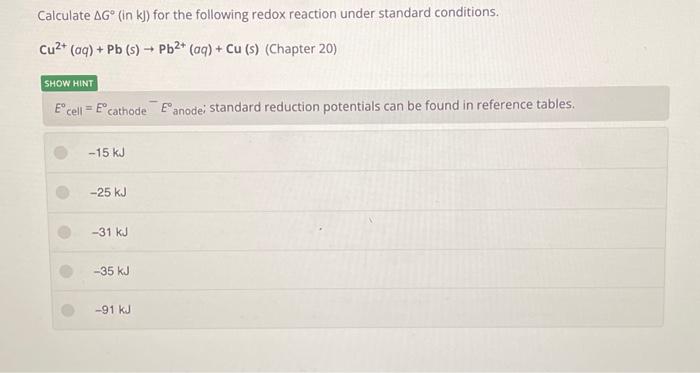
To calculate the Gibbs free energy change (?G) for the given redox reaction under standard conditions, we need to use the Nernst equation: where:n is the number of moles of electrons transferredF is Faraday's constant (96,485 C/mol or J/(V·mol)) is the standard cell potential (in volts)