Home /
Expert Answers /
Chemistry /
calculate-delta-h-text-rxn-for-the-following-reaction-mathrm-fe-2-mathrm-o-pa217
(Solved): Calculate \( \Delta H_{\text {rxn }} \) for the following reaction - \[ \mathrm{Fe}_{2} \mathrm{O}_ ...
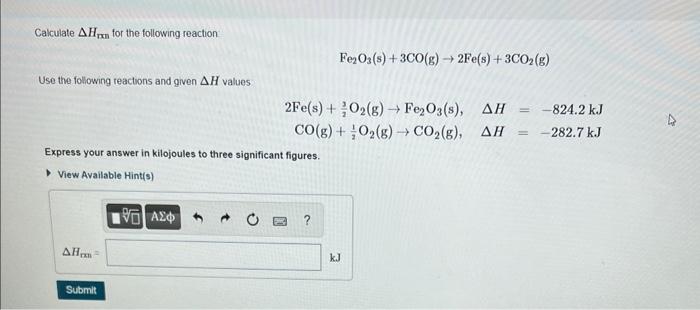
Calculate \( \Delta H_{\text {rxn }} \) for the following reaction - \[ \mathrm{Fe}_{2} \mathrm{O}_{3}(\mathrm{~s})+3 \mathrm{CO}(\mathrm{g}) \rightarrow 2 \mathrm{Fe}(\mathrm{s})+3 \mathrm{CO}_{2}(\mathrm{~g}) \] Use the following reactions and given \( \Delta H \) values \( 2 \mathrm{Fe}(\mathrm{s})+\frac{3}{2} \mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{Fe}_{2} \mathrm{O}_{3}(\mathrm{~s}), \quad \Delta H=-824.2 \mathrm{~kJ} \) \( \mathrm{CO}(\mathrm{g})+\frac{1}{2} \mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{CO}_{2}(\mathrm{~g}), \quad \Delta H=-282.7 \mathrm{~kJ} \) Express your answer in kilojoules to three significant figures.
Expert Answer
Given reaction is 2Fe(s)+ 3/2 O2(g) --> Fe2O3(s) ?H° = -824.2 KJ On reversing the equation sign o