Home /
Expert Answers /
Chemistry /
calculate-delta-g-mathrm-dmi-for-the-reaction-mathrm-mgco-3-mathrm-s-rightar-pa596
(Solved): Calculate \( \Delta G_{\mathrm{Dmi}} \) for the reaction \[ \mathrm{MgCO}_{3}(\mathrm{~s}) \rightar ...
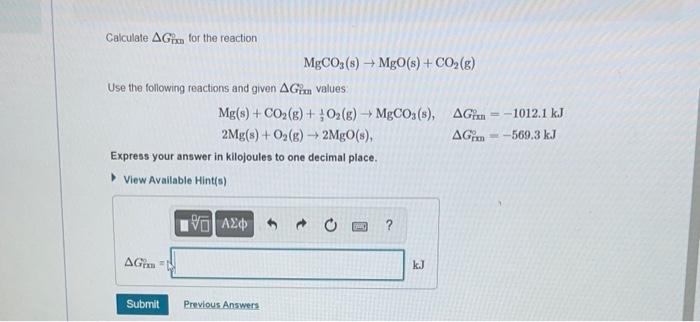
Calculate \( \Delta G_{\mathrm{Dmi}} \) for the reaction \[ \mathrm{MgCO}_{3}(\mathrm{~s}) \rightarrow \mathrm{MgO}(\mathrm{s})+\mathrm{CO}_{2}(\mathrm{~g}) \] Use the following reactions and given \( \Delta G_{\mathrm{r} \text { in }} \) values \[ \begin{array}{ll} \mathrm{Mg}(\mathrm{s})+\mathrm{CO}_{2}(\mathrm{~g})+\frac{1}{2} \mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{MgCO}_{3}(\mathrm{~s}), & \Delta G_{\mathrm{rmn}}^{\prime}=-1012.1 \mathrm{~kJ} \\ 2 \mathrm{Mg}(\mathrm{s})+\mathrm{O}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{MgO}(\mathrm{s}), & \Delta G_{\mathrm{rm}}^{\circ}=-569.3 \mathrm{~kJ} \end{array} \] Express your answer in kilojoules to one decimal place.