Home /
Expert Answers /
Chemistry /
calcium-carbonate-will-decompose-when-heated-to-produce-calcium-oxide-and-carbon-dioxide-as-shown-i-pa287
(Solved): Calcium carbonate will decompose when heated to produce calcium oxide and carbon dioxide as shown i ...
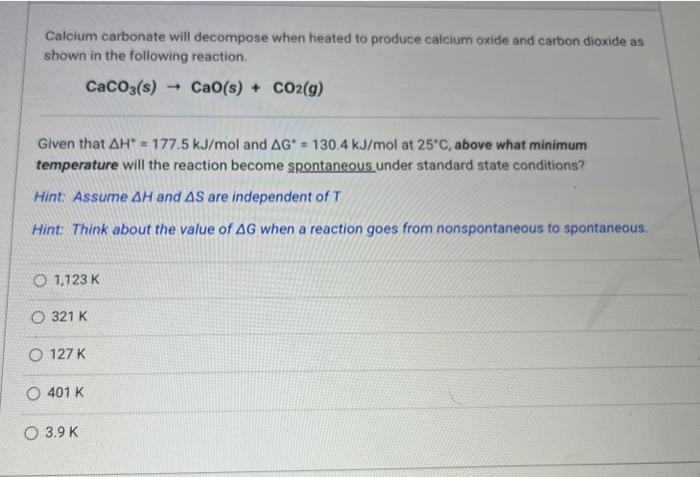
Calcium carbonate will decompose when heated to produce calcium oxide and carbon dioxide as shown in the following reaction. Given that and at , above what minimum temperature will the reaction become spontaneous under standard state conditions? Hint: Assume and are independent of Hint: Think about the value of when a reaction goes from nonspontaneous to spontaneous.