Home /
Expert Answers /
Chemistry /
bromine-monochloride-is-synthesized-using-the-reaction-left-mathrm-br-2-mathrm-g-mathr-pa162
(Solved): Bromine monochloride is synthesized using the reaction \[ \left.\mathrm{Br}_{2}(\mathrm{~g})+\mathr ...
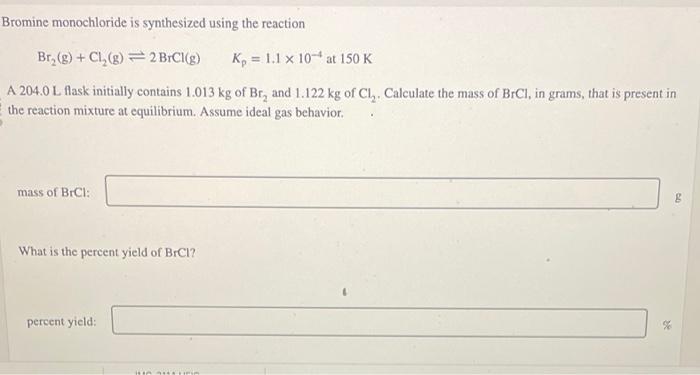
Bromine monochloride is synthesized using the reaction \[ \left.\mathrm{Br}_{2}(\mathrm{~g})+\mathrm{Cl}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{BrCl}_{\mathrm{g}}\right) \quad K_{\mathrm{p}}=1.1 \times 10^{-4} \mathrm{at} 150 \mathrm{~K} \] A 204.0 L flask initially contains \( 1.013 \mathrm{~kg} \) of \( \mathrm{Br}_{2} \) and \( 1.122 \mathrm{~kg} \) of \( \mathrm{Cl}_{2} \). Calculate the mass of \( \mathrm{BrCl} \), in grams, that is present in the reaction mixture at equilibrium. Assume ideal gas behavior. mass of \( \mathrm{BrCl} \) : What is the percent yield of \( \mathrm{BrCl} \) ? percent yield: