Home /
Expert Answers /
Chemistry /
br-2-g-ch-4-g-nano-3-nano-3-aq-nano-3-s-nano-3-aq-ch-4-g-ch-3-ch-3-g-pa420
(Solved): Br_(2)(g)CH_(4)(g)NaNO_(3)NaNO_(3)(aq)NaNO_(3)(s)NaNO_(3)(aq)CH_(4)(g)CH_(3)CH_(3)(g) ...
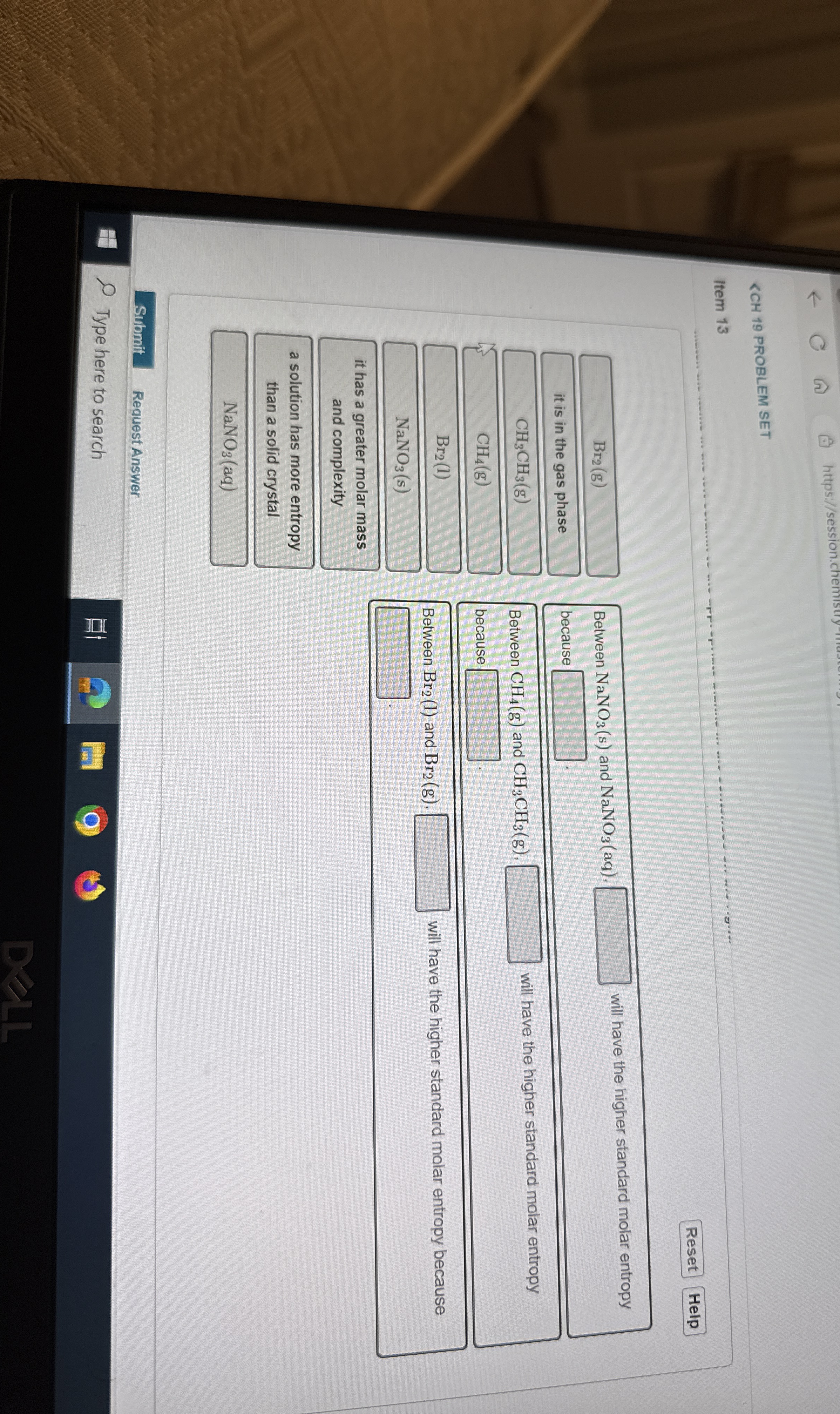
Br_(2)(g)?CH_(4)(g)??NaNO_(3)NaNO_(3)(aq)NaNO_(3)(s)NaNO_(3)(aq)?CH_(4)(g)CH_(3)CH_(3)(g)??Br_(2)(l)Br_(2)(g)??
Br_(2)(g)
it is in the gas phase
?
CH_(4)(g)
?
?
NaNO_(3) (s)
it has a greater molar mass and complexity
a solution has more entropy than a solid crystal
NaNO_(3)(aq)
Between NaNO_(3)(s) and NaNO_(3)(aq). ? will have the higher standard molar entropy
Between CH_(4)(g) and CH_(3)CH_(3)(g). ? will have the higher standard molar entropy because ?
Between Br_(2)(l) and Br_(2)(g), will have the higher standard molar entropy because
?
Submit
Request Answer
Type here to search