Home /
Expert Answers /
Chemistry /
both-from-the-first-picture-and-then-1-2-3-7-and-9-please-for-the-solubility-study-experiment-8-pa897
(Solved): both from the first picture and then 1, 2, 3, 7, and 9 please For the Solubility Study (experiment 8 ...
both from the first picture and then 1, 2, 3, 7, and 9 please






For the Solubility Study (experiment 8) we would normally come into the lab and set up acid-base titrations to be able to complete data collection and then work on analysis of that data to calculate the molarity (molar solubility) of two separate base (calcium hydroxide) solutions. From the molarity (molar solubility) we can calculate the \( K_{s p} \) for the base. We will still act as though we are performing the experiment according to the lab procedure, think through the experiment and then use class data from a previous semester. You will need to use this data to work through your lab report questions. 1) Write out the equilibrium equation for the dissolution of calcium hydroxide in water. 2) Write out the equation for the acid-base reaction (titration) of calcium hydroxide and hydrochloric acid in water.
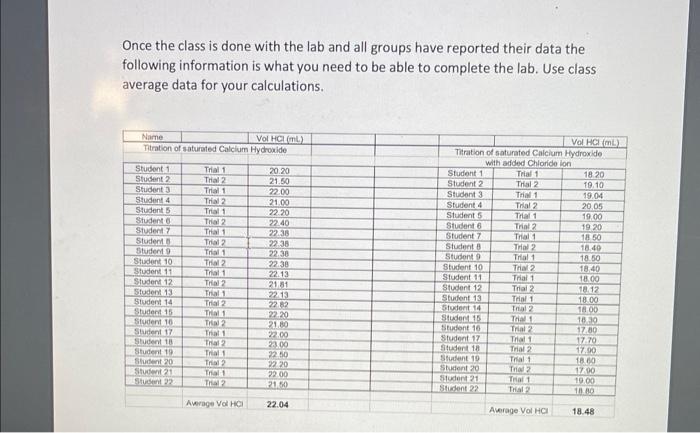
Once the class is done with the lab and all groups have reported their data the following information is what you need to be able to complete the lab. Use class average data for your calculations.
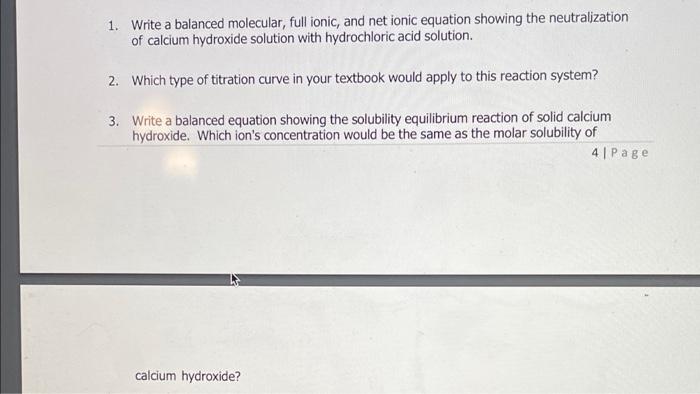
1. Write a balanced molecular, full ionic, and net ionic equation showing the neutralization of calcium hydroxide solution with hydrochloric acid solution. 2. Which type of titration curve in your textbook would apply to this reaction system? 3. Write a balanced equation showing the solubility equilibrium reaction of solid calcium hydroxide. Which ion's concentration would be the same as the molar solubility of 4 | Page calcium hydroxide?
6. Calculate the molar solubility of saturated calcium hydroxide solution and the \( \mathbf{K}_{\mathrm{sp}} \) of calcium hydroxide from your experimental data. Solution B: Saturated Calcium Hydroxide in Aqueous Calcium Chloride 7. Place about \( 100 \mathrm{~mL} \) of saturated calcium hydroxide solution containing calcium chloride from the bottle labeled "Solution B" in a dean, dry beaker. 8. Pipet \( 25.00 \mathrm{~mL} \) of "Solution B" into a clean erlenmeyer flask. Add about \( 25 \mathrm{~mL} \) of water and two or three drops of the same indicator you used in step #5. Titrate with standard hydrochloric acid solution. Repeat the titration at least once more to ensure precision. 9. Calculate the molar solubility of calcium hydroxide in "Solution B".
Expert Answer
Answer: In titrtion HCl will react with the portion of Ca(OH)2 which is in solution. That is from amount of HCl consumed in titration, we can find out amount of Ca(OH)2 which is in soluble form in the saturated solution. HCl and Ca(OH)2 react accordi