Home /
Expert Answers /
Chemistry /
blank-1-non-spontaneous-or-spontaneous-blank-2-gibbs-free-energy-entropy-enthalpy-or-temperatur-pa484
(Solved): blank 1: non-spontaneous or spontaneous blank 2: gibbs free energy, entropy, enthalpy, or temperatur ...

blank 1: non-spontaneous or spontaneous
blank 2: gibbs free energy, entropy, enthalpy, or temperature
blank 3: less than, equal to, or greater than

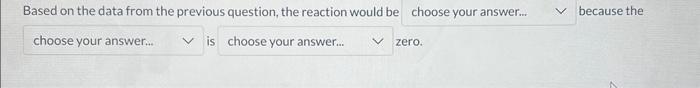
Based on the data from the previous question, the reaction would be because the is zero.
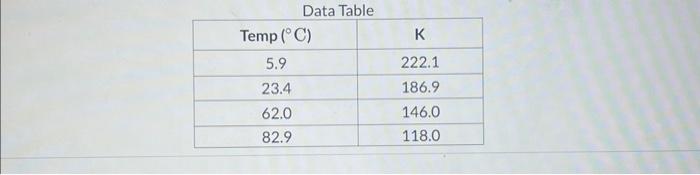
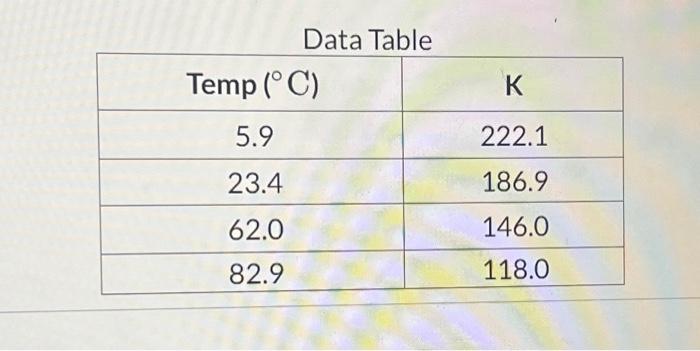
In lab a student calculates the following equilibrium concentrations for at each of the specified temperatures. Calculate in units of . Report your answer with two places after the decimal.
Data Table \begin{tabular}{|c|c|} \hline Temp & \\ \hline 5.9 & 222.1 \\ \hline 23.4 & 186.9 \\ \hline 62.0 & 146.0 \\ \hline 82.9 & 118.0 \\ \hline \end{tabular}
Data Table \begin{tabular}{|c|c|} \hline & \\ \hline 5.9 & 222.1 \\ \hline 23.4 & 186.9 \\ \hline 62.0 & 146.0 \\ \hline 82.9 & 118.0 \\ \hline \end{tabular}
In lab a student calculates the following equilibrium concentrations for at each of the specified temperatures: Calculate in units of . Report your answer with two places after the decimal.
Expert Answer
The Van 't Hoff equation gives us information about the change in the equilibrium constant, , of ...