Home /
Expert Answers /
Chemistry /
benzoic-acid-dissociates-in-water-to-form-hydrogen-ions-and-benzoate-ions-this-reaction-is-an-ondo-pa895
(Solved): Benzoic acid dissociates in water to form hydrogen ions and benzoate ions. This reaction is an ondo ...

Benzoic acid dissociates in water to form hydrogen ions and benzoate ions. This reaction is an ondothermic reaction: \[ \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COOH}(\mathrm{aq}) \rightleftharpoons \mathrm{CeH}_{5} \mathrm{COO}^{-}(\mathrm{sq})+\mathrm{H}^{+}(\mathrm{aq}) \] Suppose this reaction is at equitbrium. The following changes (stresses) will shitt the equilibrium toward the product side or toward the reactant bide. Classity ench of the changes according to which side of the reaction is expected to shit due to the applied change. Drag the appropriate ltems to their respective bins. View Available Hint(s) Equilbrium shitts toward reactants Equilibrium shifts toward products
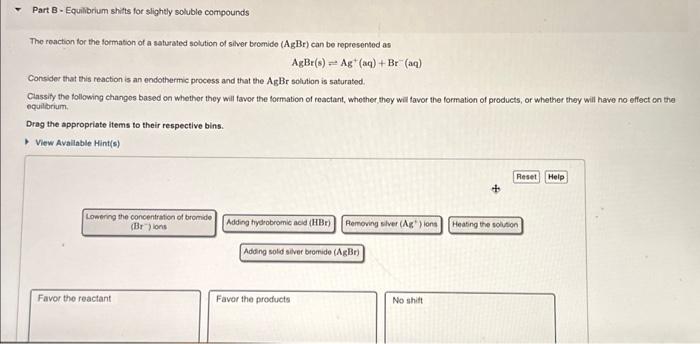
The rosction for the formation of a saturated solution of silver bromido \( (\mathrm{AgBr}) \) can be represented as \[ \mathrm{AgBr}(\mathrm{s}) \rightleftharpoons \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{Br}^{-}(\mathrm{aq}) \] Consider that this reaction is an endothermic process and that the \( \mathrm{AgBr} \) solution is saturated. Ciassity the following changos based on whether they will favor the formation of reactant, whother they will favor the formation of products, of whether they will have no effect on the equiliorium. Drag the appropriate items to their respective bins. View Avallable Hint(s) Lowerng the concentration of bromide (Br 7 )ions
Expert Answer
Answer Part A According to Le chatliers principle, when external stress is applied on a system at equilibrium, the system shifts the position of equilibrium so as to nullify the effect of stress. 1)When H+ is removed by adding NaOH, the concentration