Home /
Expert Answers /
Chemistry /
below-is-a-schematic-representation-of-the-molecules-for-forming-a-solution-of-the-solid-biphenyl-pa703
(Solved): Below is a schematic representation of the molecules for forming a solution of the solid biphenyl ...
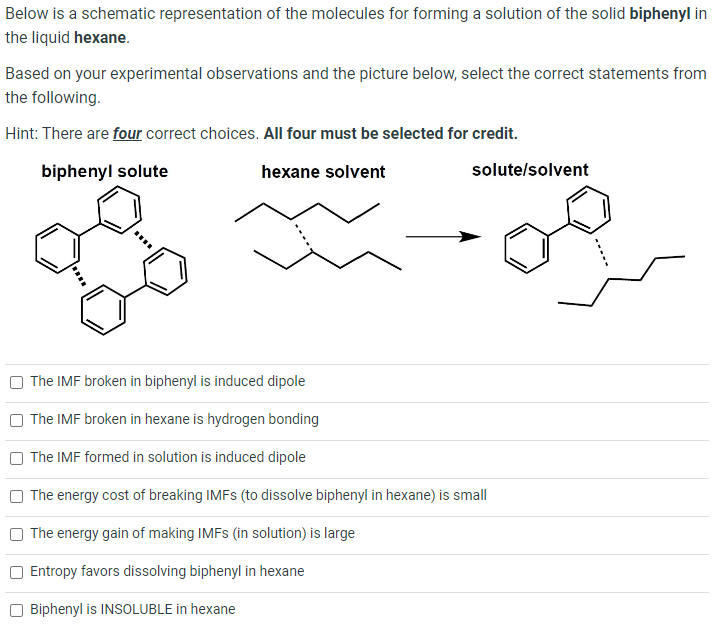
Below is a schematic representation of the molecules for forming a solution of the solid biphenyl in the liquid hexane. Based on your experimental observations and the picture below, select the correct statements from the following. Hint: There are four correct choices. All four must be selected for credit. biphenyl solute hexane solvent solute/solvent The IMF broken in biphenyl is induced dipole The IMF broken in hexane is hydrogen bonding The IMF formed in solution is induced dipole The energy cost of breaking IMFs (to dissolve biphenyl in hexane) is small The energy gain of making IMFs (in solution) is large Entropy favors dissolving biphenyl in hexane Biphenyl is INSOLUBLE in hexane