Home /
Expert Answers /
Chemistry /
begin-array-ccc-mathrm-c-6-mathrm-h-6-mathrm-o-6-2-mathrm-h-2-mathrm-e-pa194
(Solved): \[ \begin{array}{ccc} \mathrm{C}_{6} \mathrm{H}_{6} \mathrm{O}_{6}+2 \mathrm{H}^{+}+2 \mathrm{e}^{ ...
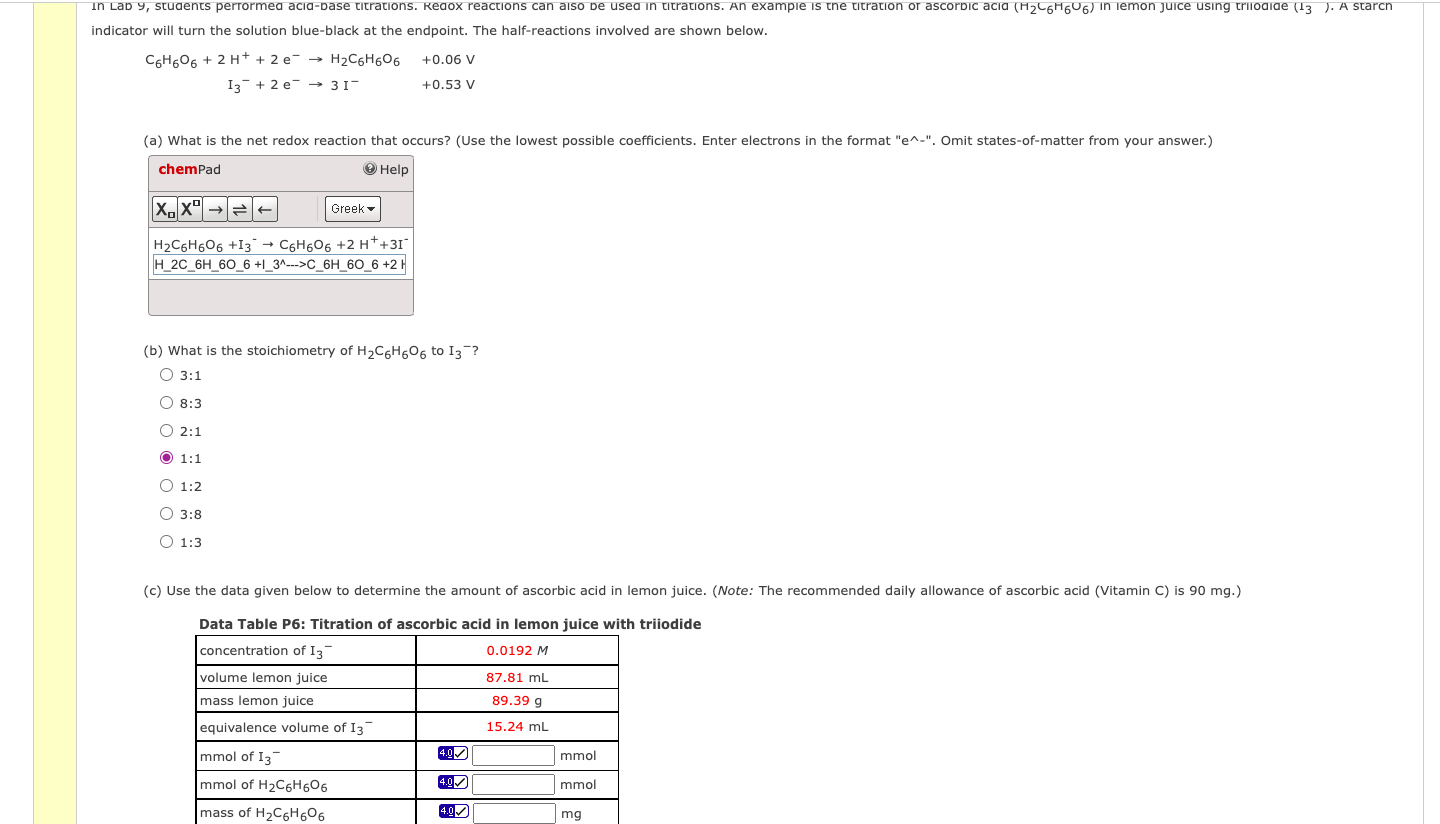
\[ \begin{array}{ccc} \mathrm{C}_{6} \mathrm{H}_{6} \mathrm{O}_{6}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-} & \rightarrow \mathrm{H}_{2} \mathrm{C}_{6} \mathrm{H}_{6} \mathrm{O}_{6} & +0.06 \mathrm{~V} \\ \mathrm{I}_{3}^{-}+2 \mathrm{e}^{-} \rightarrow 3 \mathrm{I}^{-} & +0.53 \mathrm{~V} \end{array} \] (a) What is the net redox reaction that occurs? (Use the lowest possible coefficients. Enter electrons in the format "e^-". Omit states-of-matter from your answer.) (b) What is the stoichiometry of \( \mathrm{H}_{2} \mathrm{C}_{6} \mathrm{H}_{6} \mathrm{O}_{6} \) to \( \mathrm{I}_{3}{ }^{-} \)? \[ \begin{array}{l} 3: 1 \\ 8: 3 \\ 2: 1 \\ 1: 1 \\ 1: 2 \\ 3: 8 \\ 1: 3 \end{array} \] (c) Use the data given below to determine the amount of ascorbic acid in lemon juice. (Note: The recommended daily allowance of ascorbic acid (Vitamin C) is 90 mg.) Data Table P6: Titration of ascorbic acid in lemon juice with triiodide