Home /
Expert Answers /
Chemistry /
based-on-their-lewis-structures-predict-the-ordering-of-the-mathrm-o-mathrm-o-bond-lengt-pa890
(Solved): Based on their Lewis structures, predict the ordering of the \( \mathrm{O}-\mathrm{O} \) bond lengt ...
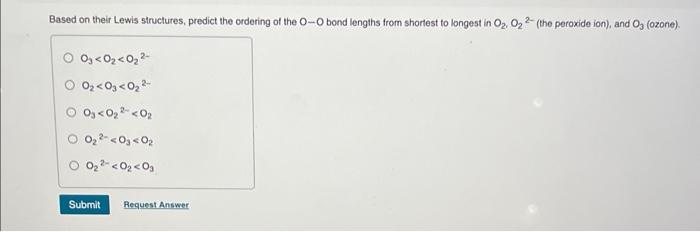
Based on their Lewis structures, predict the ordering of the \( \mathrm{O}-\mathrm{O} \) bond lengths from shortest to longest in \( \mathrm{O}_{2}, \mathrm{O}_{2}{ }^{2-} \) (the peroxide ion), and \( \mathrm{O}_{3} \) (ozone). \[ \begin{array}{l} \mathrm{O}_{3}<\mathrm{O}_{2}<\mathrm{O}_{2}{ }^{2-} \\ \mathrm{O}_{2}<\mathrm{O}_{3}<\mathrm{O}_{2}{ }^{2-} \\ \mathrm{O}_{3}<\mathrm{O}_{2}{ }^{2-}<\mathrm{O}_{2} \\ \mathrm{O}_{2}{ }^{2-}<\mathrm{O}_{3}<\mathrm{O}_{2} \\ \mathrm{O}_{2}{ }^{2-}<\mathrm{O}_{2}<\mathrm{O}_{3} \end{array} \]
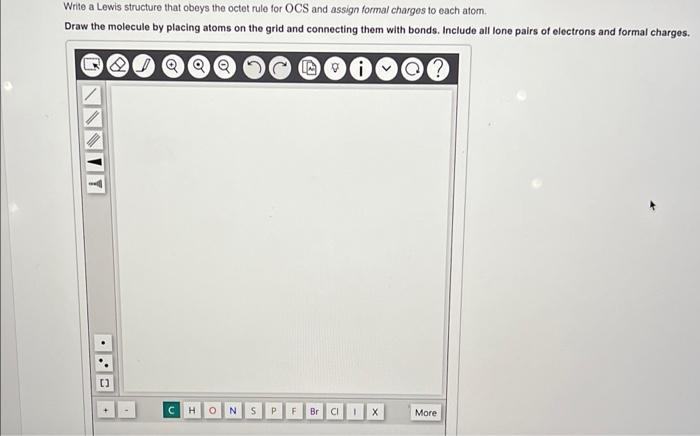
Write a Lewis structure that obeys the octet rule for OCS and assign formal charges to each atom. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons and formal charges.
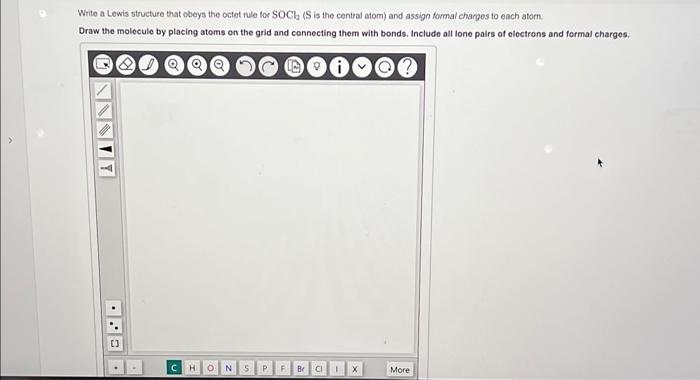
Write a Lewis structure that obeys the octet rule tor \( \mathrm{SOCl}_{2} \) ( \( \mathrm{S} \) is the central afom) and assign formal charges to each atom. Draw the molecule by placing atoms on the grid and connecting them with bonds, Include all lone pairs of electrons and formal charges.
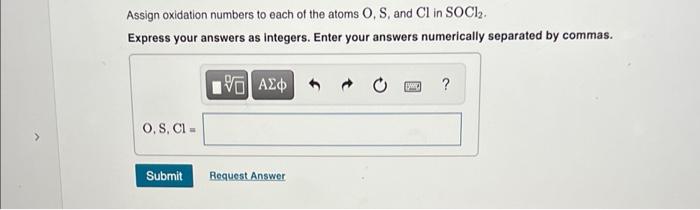
Assign oxidation numbers to each of the atoms \( \mathrm{O}, \mathrm{S} \), and \( \mathrm{Cl} \) in \( \mathrm{SOCl}_{2} \). Express your answers as integers. Enter your answers numerically separated by commas.
Expert Answer
ANSWER 1 ) Options ( 2 ) is correct O2 < O3 < O2 2- Lewis structure of all compound electronic c