Home /
Expert Answers /
Chemistry /
based-on-the-sign-of-the-standard-cell-potential-e-text-cell-classify-these-reactions-pa518
(Solved): Based on the sign of the standard cell potential, \( E_{\text {cell }} \), classify these reactions ...
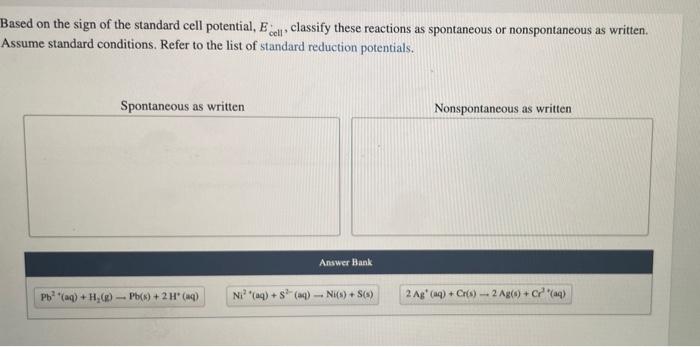
Based on the sign of the standard cell potential, \( E_{\text {cell }} \), classify these reactions as spontaneous or nonspontaneous as written. Assume standard conditions. Refer to the list of standard reduction potentials.
Expert Answer
. we need to check spontaneity of reactions, we