Home /
Expert Answers /
Chemistry /
balance-the-following-equation-and-select-the-correct-list-of-coefficients-mathrm-c-7-math-pa802
(Solved): Balance the following equation and select the correct list of coefficients: \( \mathrm{C}_{7} \math ...
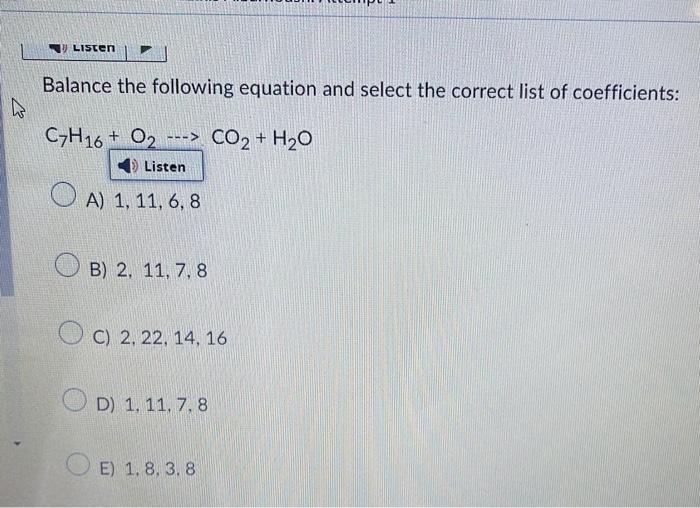
Balance the following equation and select the correct list of coefficients: \( \mathrm{C}_{7} \mathrm{H}_{16}+\mathrm{O}_{2} \cdots \mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O} \) A) \( 1,11,6,8 \) B) \( 2,11,7,8 \) C) \( 2,22,14,16 \) D) \( 1,11,7,8 \) E) \( 1,8,3,8 \)
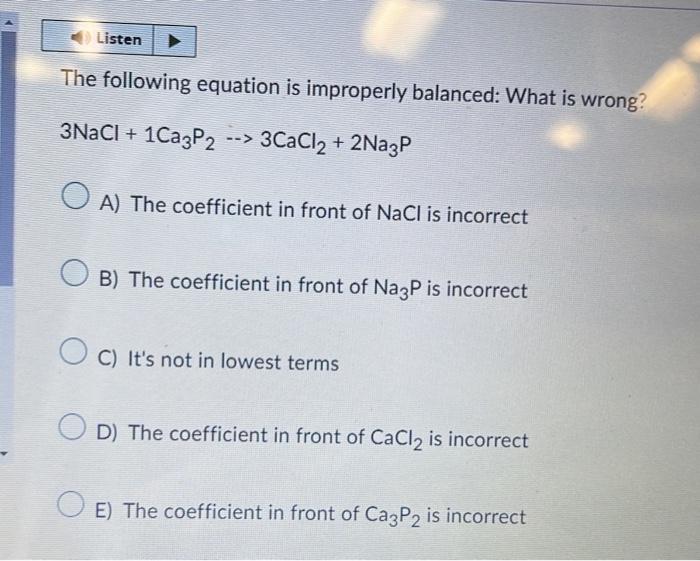
The following equation is improperly balanced: What is wrong? \[ 3 \mathrm{NaCl}+1 \mathrm{Ca}_{3} \mathrm{P}_{2} \rightarrow 3 \mathrm{CaCl}_{2}+2 \mathrm{Na}_{3} \mathrm{P} \] A) The coefficient in front of \( \mathrm{NaCl} \) is incorrect B) The coefficient in front of \( \mathrm{Na}_{3} \mathrm{P} \) is incorrect C) It's not in lowest terms D) The coefficient in front of \( \mathrm{CaCl}_{2} \) is incorrect E) The coefficient in front of \( \mathrm{Ca}_{3} \mathrm{P}_{2} \) is incorrect
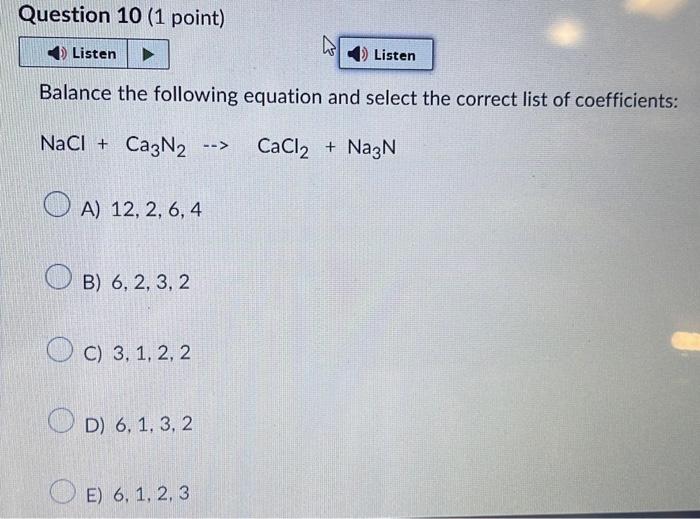
Balance the following equation and select the correct list of coefficients: \( \mathrm{NaCl}+\mathrm{Ca}_{3} \mathrm{~N}_{2} \rightarrow \mathrm{CaCl}_{2}+\mathrm{Na}_{3} \mathrm{~N} \) A) \( 12,2,6,4 \) B) \( 6,2,3,2 \) C) \( 3,1,2,2 \) D) \( 6,1,3,2 \) E) \( 6,1,2,3 \)
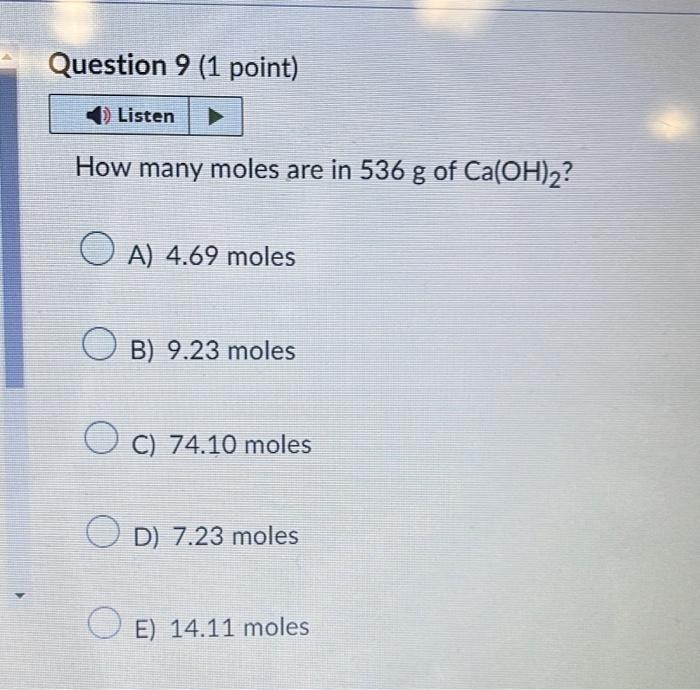
How many moles are in \( 536 \mathrm{~g} \) of \( \mathrm{Ca}(\mathrm{OH})_{2} \) ? A) \( 4.69 \) moles B) \( 9.23 \) moles C) \( 74.10 \) moles D) \( 7.23 \) moles E) \( 14.11 \) moles