Home /
Expert Answers /
Mechanical Engineering /
b-consider-an-empty-aluminium-time-capsule-as-depicted-in-figure-3-below-figure-3-depiction-of-pa283
(Solved): b. Consider an empty aluminium time-capsule, as depicted in Figure 3 below. Figure 3 Depiction of ...
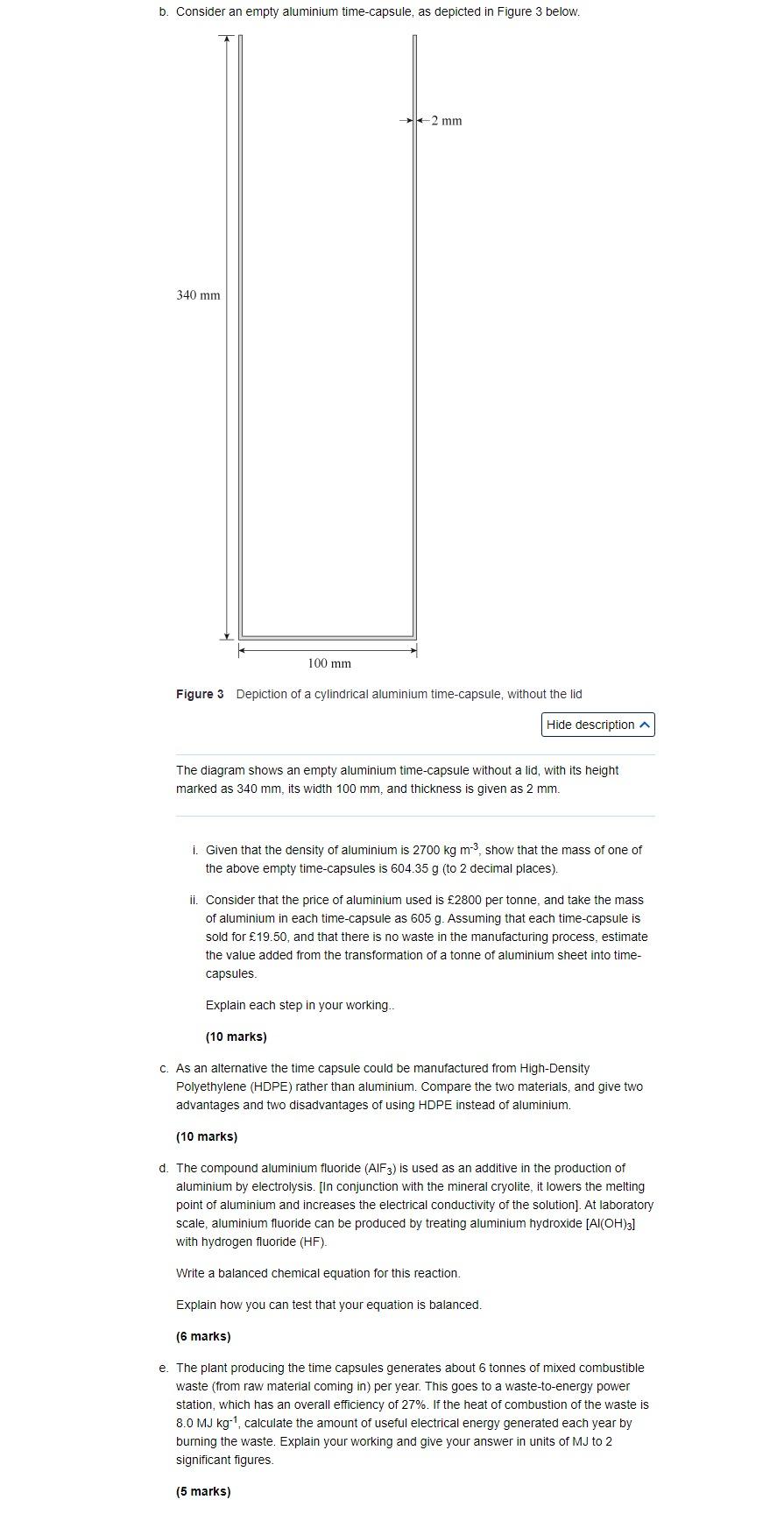
b. Consider an empty aluminium time-capsule, as depicted in Figure 3 below. Figure 3 Depiction of a cylindrical aluminium time-capsule, without the lid The diagram shows an empty aluminium time-capsule without a lid, with its height marked as \( 340 \mathrm{~mm} \), its width \( 100 \mathrm{~mm} \), and thickness is given as \( 2 \mathrm{~mm} \). i. Given that the density of aluminium is \( 2700 \mathrm{~kg} \mathrm{~m}^{-3} \), show that the mass of one of the above empty time-capsules is \( 604.35 \mathrm{~g} \) (to 2 decimal places). ii. Consider that the price of aluminium used is \( £ 2800 \) per tonne, and take the mass of aluminium in each time-capsule as \( 605 \mathrm{~g} \). Assuming that each time-capsule is sold for \( £ 19.50 \), and that there is no waste in the manufacturing process, estimate the value added from the transformation of a tonne of aluminium sheet into timecapsules. Explain each step in your working.. (10 marks) c. As an alternative the time capsule could be manufactured from High-Density Polyethylene (HDPE) rather than aluminium. Compare the two materials, and give two advantages and two disadvantages of using HDPE instead of aluminium. (10 marks) d. The compound aluminium fluoride \( \left(\mathrm{AlF}_{3}\right) \) is used as an additive in the production of aluminium by electrolysis. [In conjunction with the mineral cryolite, it lowers the melting point of aluminium and increases the electrical conductivity of the solution]. At laboratory scale, aluminium fluoride can be produced by treating aluminium hydroxide \( \left[\mathrm{Al}(\mathrm{OH})_{3}\right] \) with hydrogen fluoride (HF). Write a balanced chemical equation for this reaction. Explain how you can test that your equation is balanced. (6 marks) e. The plant producing the time capsules generates about 6 tonnes of mixed combustible waste (from raw material coming in) per year. This goes to a waste-to-energy power station, which has an overall efficiency of \( 27 \% \). If the heat of combustion of the waste is 8.0 \( \mathrm{MJ} \mathrm{kg}^{-1} \), calculate the amount of useful electrical energy generated each year by burning the waste. Explain your working and give your answer in units of MJ to 2 significant figures. (5 marks)
Expert Answer
Sol.- b. i. Length of capsule, L = 340 mm = 0.34 m Diameter, D = 100 mm, radius, R = 50 mm = 0.05 m Thickness, t = 2 mm Density of aluminum, = 2700 kg/m3. Internal radius, r = R - t = 50 - 2 = 48 mm = 0.048 m Internal length, l = L - t = 340 - 2 = 33