Home /
Expert Answers /
Advanced Math /
b-calculate-the-equilibrium-constant-of-the-redox-reaction-between-the-two-redox-couples-2p-e-s-pa432
(Solved): B. Calculate the equilibrium constant of the redox reaction between The two redox couples (2p) E S ...
B. Calculate the equilibrium constant of the redox reaction between The two redox couples (2p) E° S2O82-/2SO4²= +2.08V E° Fe³+ /Fe²+ = +0.77V
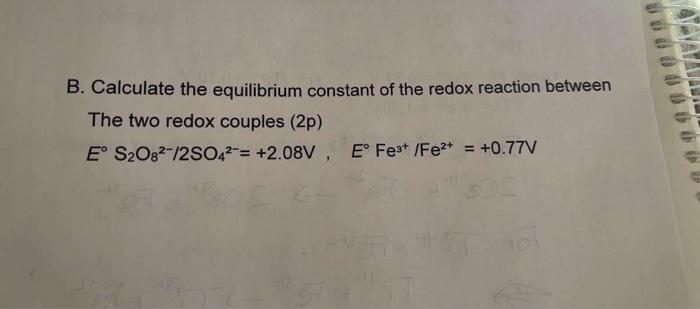
B. Calculate the equilibrium constant of the redox reaction between The two redox couples