Home /
Expert Answers /
Chemistry /
automotive-air-bags-inflate-when-a-sample-of-sodium-azide-nan3-decomposes-rapidly-via-the-followin-pa803
(Solved): Automotive air bags inflate when a sample of sodium azide, NaN3, decomposes rapidly via the followin ...
Automotive air bags inflate when a sample of sodium azide, NaN3, decomposes rapidly via the following chemical reaction: 2 NaN3(s) ??? 2 Na(s) + 3 N?(g) - What mass (in kg) of sodium azide would you need to produce XL of nitrogen gas? The density of nitrogen gas is Z g/L.
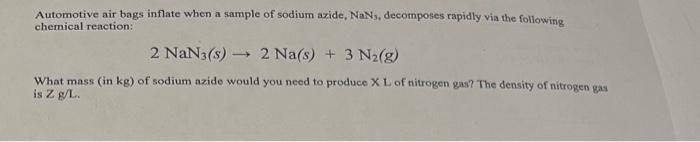
Automotive air bags inflate when a sample of sodium azide, , decomposes rapidly via the following chemical reaction: What mass (in ) of sodium azide would you need to produce X L of nitrogen gas? The density of nitrogen is .