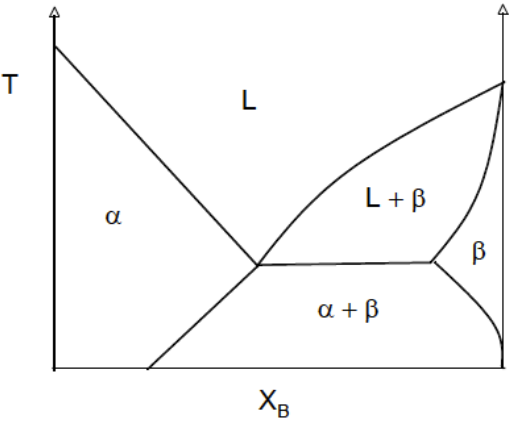
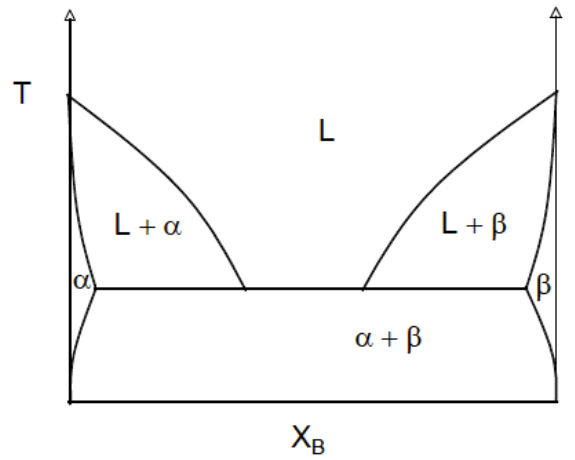
(Solved): Assume there are two hypothetical phase diagrams as depicted below. Using Gibbs free energy vs. comp ...
Assume there are two hypothetical phase diagrams as depicted below. Using Gibbs free energy vs. composition curves (supplemented with explanations), show that the following two phase diagrams cannot be formed.
- A solid phase (alpha) which has a continuous boundary with the liquid phase but is not accompanied by the existence of two solid-liquid phases (alpha + liquid).
- Phase diagram with a wide boundary between the liquid phase and the separate alpha/beta solid phase (phase-separated alpha/beta solids).