Home /
Expert Answers /
Mechanical Engineering /
as-shown-in-the-figure-below-a-gas-contained-within-a-piston-cylinder-assembly-initially-at-a-vol-pa190
(Solved): As shown in the figure below, a gas contained within a piston-cylinder assembly, initially at a vol ...
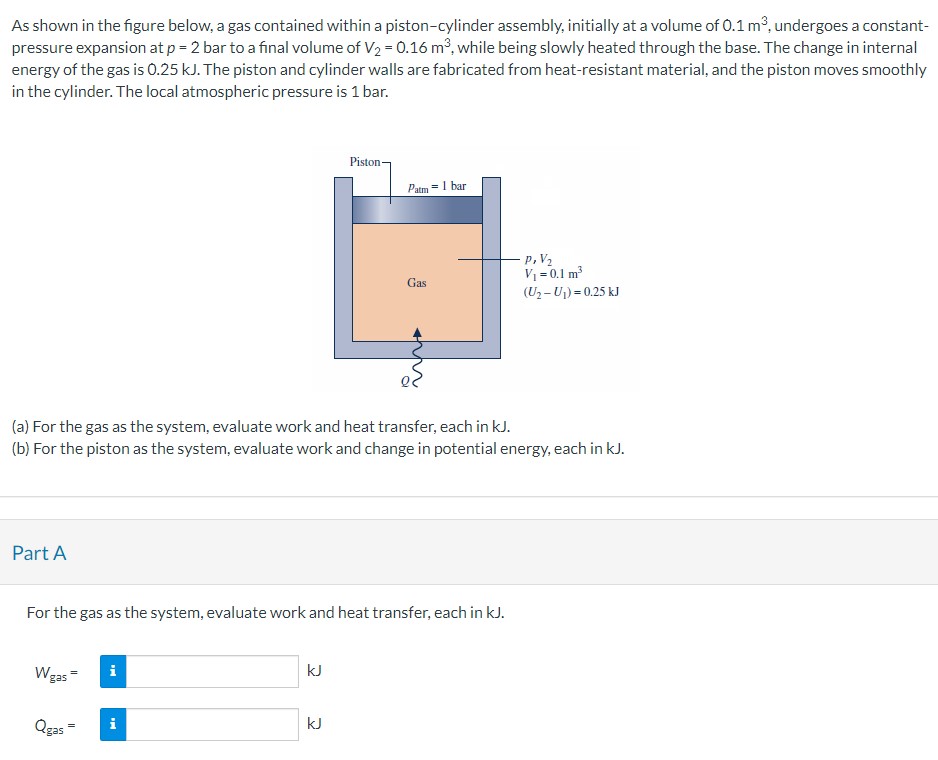
As shown in the figure below, a gas contained within a piston-cylinder assembly, initially at a volume of , undergoes a constantpressure expansion at bar to a final volume of , while being slowly heated through the base. The change in internal energy of the gas is . The piston and cylinder walls are fabricated from heat-resistant material, and the piston moves smoothly in the cylinder. The local atmospheric pressure is 1 bar. (a) For the gas as the system, evaluate work and heat transfer, each in . (b) For the piston as the system, evaluate work and change in potential energy, each in . Part A For the gas as the system, evaluate work and heat transfer, each in .