Home /
Expert Answers /
Chemistry /
are-there-the-cirrect-calculations-report-sheet-spectrophotometry-food-dyes-part-a-data-table-1-pa211
(Solved): are there the cirrect calculations Report Sheet: Spectrophotometry \& Food Dyes Part A: Data Table 1 ...
are there the cirrect calculations
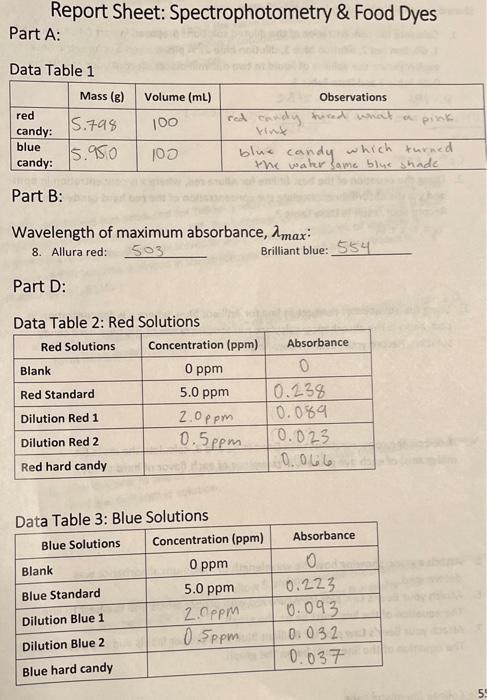



Report Sheet: Spectrophotometry \& Food Dyes Part A: Data Table 1 Part B: Wavelength of maximum absorbance, \( \lambda_{\max } \) : 8. Allura red: 503 Brilliant blue: 554 Part D: Data Table 2: Red Solutions Tahlo 2. Rhis Solutions
C. Prepare Standard Solutions for Each Food Dye 1. Obtain about \( 100 \mathrm{~mL} \) of the \( 5 \mathrm{ppm} \) standard solution for Allura red. 2. Label 2 beakers dilution Red 1 \& dilution Red 2 . 3. Pipet \( 10.00 \mathrm{~mL} \) of standard solution into a \( 25.00-\mathrm{mL} \) volumetric flask and fill to the line with distilled water. If you do not pipet exactly \( 10.00 \mathrm{~mL} \), you will need to record the actual volume used. 4. Pour the solution into the beaker labeled dilution Red 1. 5. Pipet \( 10.00 \mathrm{~mL} \) of standard solution into a100.00-mL volumetric flask and fill to the line with distilled water. If you do not pipet exactly \( 10.00 \mathrm{~mL} \), you will need to record the actual volume used. 6. Pour the solution into the beaker labeled dilution Red 2 . 7. Thoroughly rinse the volumetric flasks with water. Do not clean with soap. Do a final rinse with DI water. 8. Obtain about \( 100 \mathrm{~mL} \) of the \( 5 \mathrm{ppm} \) standard solution for Brilliant Blue. 9. Label two beakers dilution Blue 1 \& dilution Blue 2 . 10. Pipet \( 10.00 \mathrm{~mL} \) of standard solution into a \( 25.00-\mathrm{mL} \) volumetric flask and fill to the line with distilled water. If you do not pipet exactly \( 10.00 \mathrm{~mL} \), you will need to record the actual volume used. 11. Pour the solution into the beaker labeled dilution Blue 1. 12. Pipet \( 10.00 \mathrm{~mL} \) of standard solution into a \( 100.00-\mathrm{mL} \) volumetric flask and fill to the line with distilled water. If you do not pipet exactly \( 10.00 \mathrm{~mL} \), you will need to record the actual volume used. 13. Pour the solution into the beaker labeled dilution Blue 2 . 14. Thoroughly rinse the volumetric flasks with water. Do not clean with soap. Do a final rinse with DI water.
Expert Answer
Solution: Part D. Red solutions: Dilute solution concentration = (Standard solution concentration x Standard solutio