Home /
Expert Answers /
Chemistry /
aqueous-solutions-of-sodium-bicarbonate-and-sulfuric-acid-react-to-produce-carbon-dioxide-according-pa607
(Solved): Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according ...
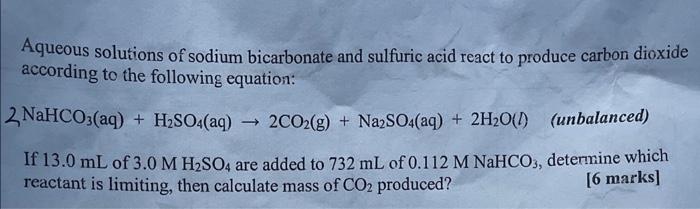
Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: If of are added to of , determine which reactant is limiting, then calculate mass of produced? [6 marks]