Home /
Expert Answers /
Chemistry /
aqueous-potassium-hydroxide-koh-reacts-with-aqueous-phosphoric-acid-h3po-to-form-aqueous-pa574
(Solved): Aqueous Potassium hydroxide (KOH) reacts with aqueous phosphoric acid (H3PO) to form aqueous ...
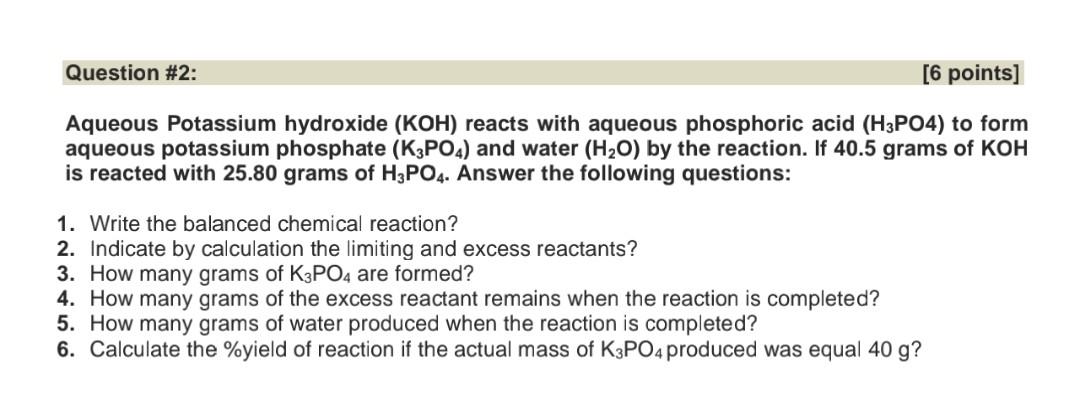
Aqueous Potassium hydroxide reacts with aqueous phosphoric acid to form aqueous potassium phosphate and water by the reaction. If 40.5 grams of is reacted with grams of . Answer the following questions: 1. Write the balanced chemical reaction? 2. Indicate by calculation the limiting and excess reactants? 3. How many grams of are formed? 4. How many grams of the excess reactant remains when the reaction is completed? 5. How many grams of water produced when the reaction is completed? 6. Calculate the \%yield of reaction if the actual mass of produced was equal ?