Home /
Expert Answers /
Chemistry /
an-unknown-element-x-has-the-following-isotopes-25x-80-50-abundant-mass-25-03-amu-and-27x-19-pa365
(Solved): An unknown element X has the following isotopes: 25X (80.50% abundant, mass = 25.03 amu) and 27X(19. ...
An unknown element X has the following isotopes: 25X (80.50% abundant, mass = 25.03 amu) and 27X
(19.50% abundant, mass = 26.98 amu). What is the average atomic mass of X in amu?
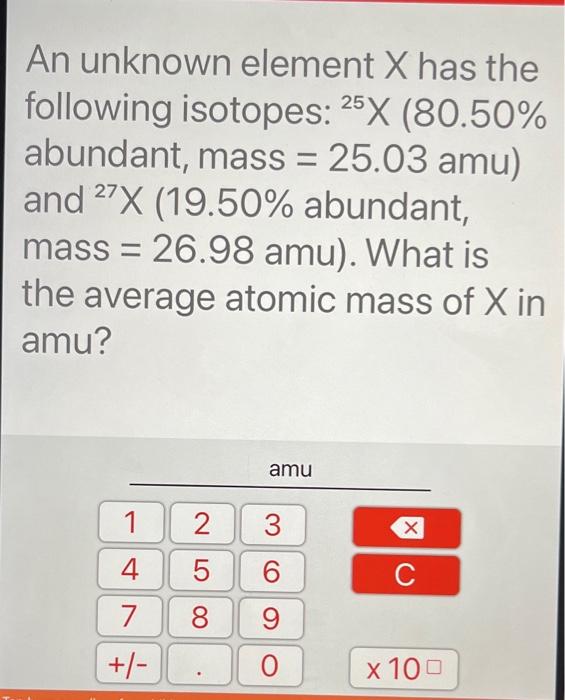
An unknown element \( X \) has the following isotopes: \( { }^{25} \mathrm{X}(80.50 \% \) abundant, mass \( =25.03 \mathrm{amu} \) ) and \( { }^{27} \mathrm{X}(19.50 \% \) abundant, mass \( =26.98 \mathrm{amu}) \). What is the average atomic mass of \( X \) in amu?