Home /
Expert Answers /
Physics /
an-insulated-beaker-with-negligible-mass-contains-liquid-water-with-a-mass-of-0-210kg-and-pa933
(Solved): An insulated beaker with negligible mass contains liquid water with a mass of 0.210kg and ...
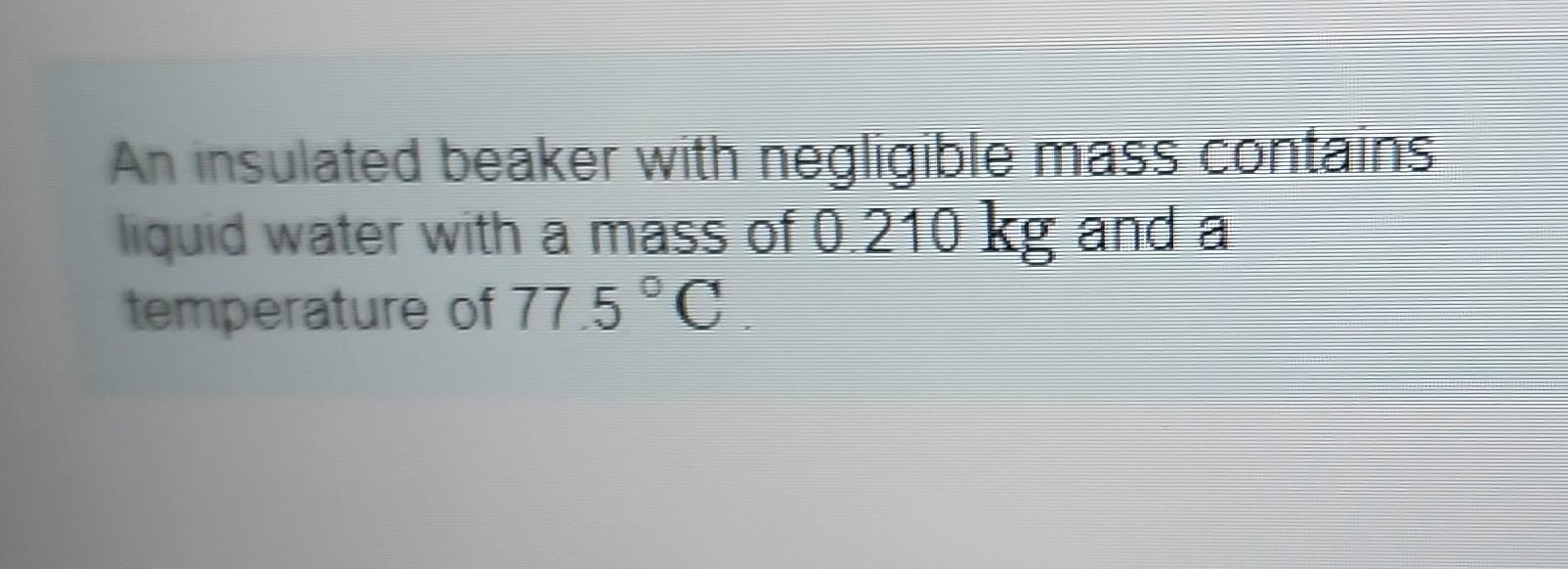

An insulated beaker with negligible mass contains liquid water with a mass of and a temperature of .
How much ice at a temperature of must be dropped into the water so that the final temperature of the system will be 30.0 'C ? Take the specific heat of liquid water to be , the specific heat of ice to be , and the heat of fusion for water to be .
Expert Answer
Mass of the water is It's temperature is Initial temperature of ice is Final temperature of th...