Home /
Expert Answers /
Chemistry /
an-electrochemical-cell-is-based-on-the-following-two-half-reactions-part-a-mathrm-ox-math-pa502
(Solved): An electrochemical cell is based on the following two half-reactions: Part A \( \mathrm{Ox}: \math ...
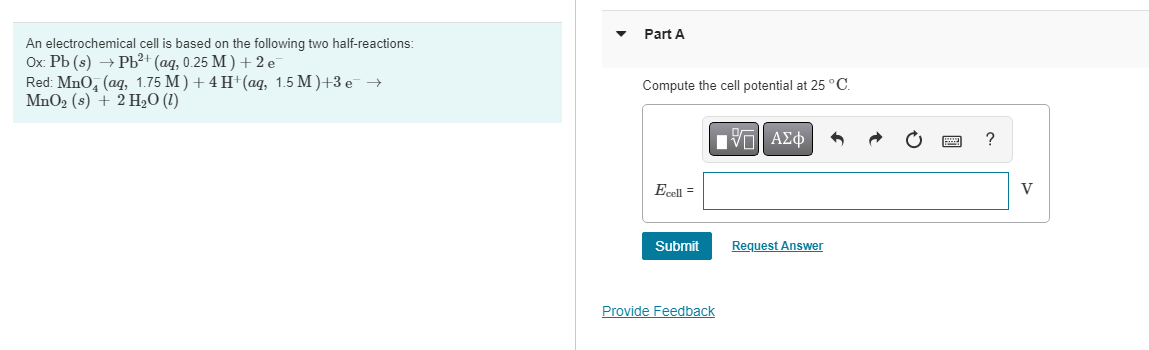
An electrochemical cell is based on the following two half-reactions: Part A \( \mathrm{Ox}: \mathrm{Pb}(s) \rightarrow \mathrm{Pb}^{2+}(a q, 0.25 \mathrm{M})+2 \mathrm{e}^{-} \) Red: \( \mathrm{MnO}_{4}^{-}(a q, 1.75 \mathrm{M})+4 \mathrm{H}^{+}(a q, 1.5 \mathrm{M})+3 \mathrm{e}^{-} \rightarrow \) Compute the cell potential at \( 25^{\circ} \mathrm{C} \). \( \mathrm{MnO}_{2}(s)+2 \mathrm{H}_{2} \mathrm{O}(l) \)