Home /
Expert Answers /
Chemistry /
an-aqueous-solution-of-hydroiodic-acid-is-standardized-by-titration-with-a-0-200m-solution-of-calci-pa855
(Solved): An aqueous solution of hydroiodic acid is standardized by titration with a 0.200M solution of calci ...


An aqueous solution of hydroiodic acid is standardized by titration with a solution of calcium hydroxide. If of base are required to neutralize of the acid, what is the molarity of the hydroiodic acid solution? 4 hydroiodic acid 9 more group attempts remaining
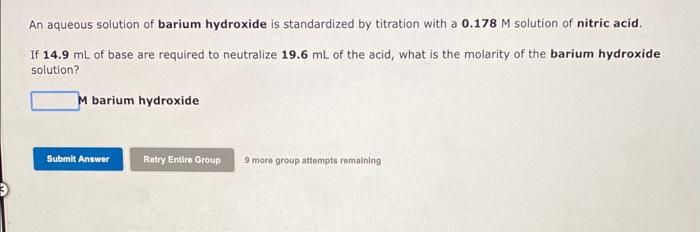
An aqueous solution of barium hydroxide is standardized by titration with a solution of nitric acid. If of base are required to neutralize of the acid, what is the molarity of the barium hydroxide solution? 4 barium hydroxide
An aqueous solution of calcium hydroxide is standardized by titration with a solution of hydrochloric acid. If of base are required to neutralize of the acid, what is the molarity of the calcium hydroxide solution? 4 calcium hydroxide