Home /
Expert Answers /
Chemistry /
an-aqueous-solution-contains-0-26m-hypochlorous-acid-one-liter-of-this-solution-could-be-converte-pa426
(Solved): An aqueous solution contains 0.26M hypochlorous acid. One Liter of this solution could be converte ...
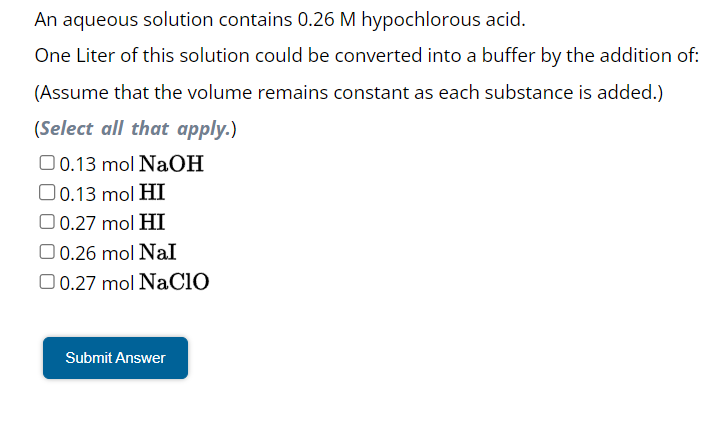
An aqueous solution contains hypochlorous acid. One Liter of this solution could be converted into a buffer by the addition of: (Assume that the volume remains constant as each substance is added.) (Select all that apply.)
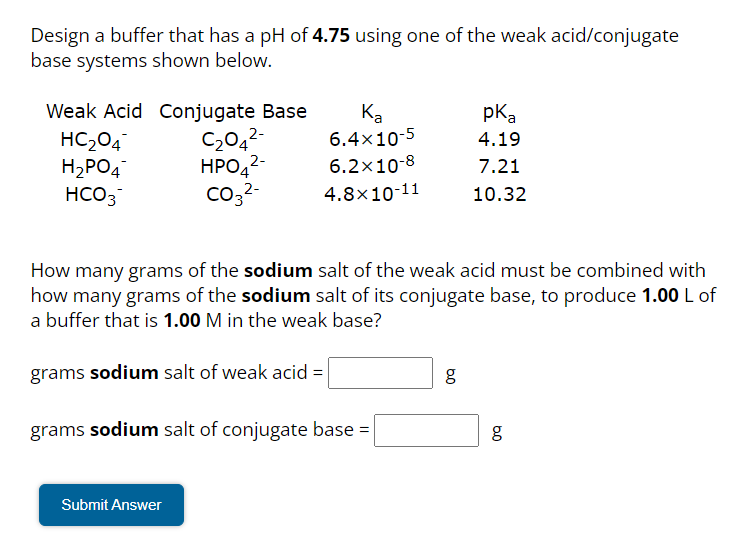
Design a buffer that has a pH of using one of the weak acid/conjugate base systems shown below. How many grams of the sodium salt of the weak acid must be combined with how many grams of the sodium salt of its conjugate base, to produce of a buffer that is in the weak base? grams sodium salt of weak acid g grams sodium salt of conjugate base g