Home /
Expert Answers /
Chemistry /
an-aqueous-kno3-solution-is-made-using-60-1g-of-kno3-diluted-to-a-total-solution-volum-pa345
(Solved): An aqueous KNO3 solution is made using 60.1g of KNO3 diluted to a total solution volum ...
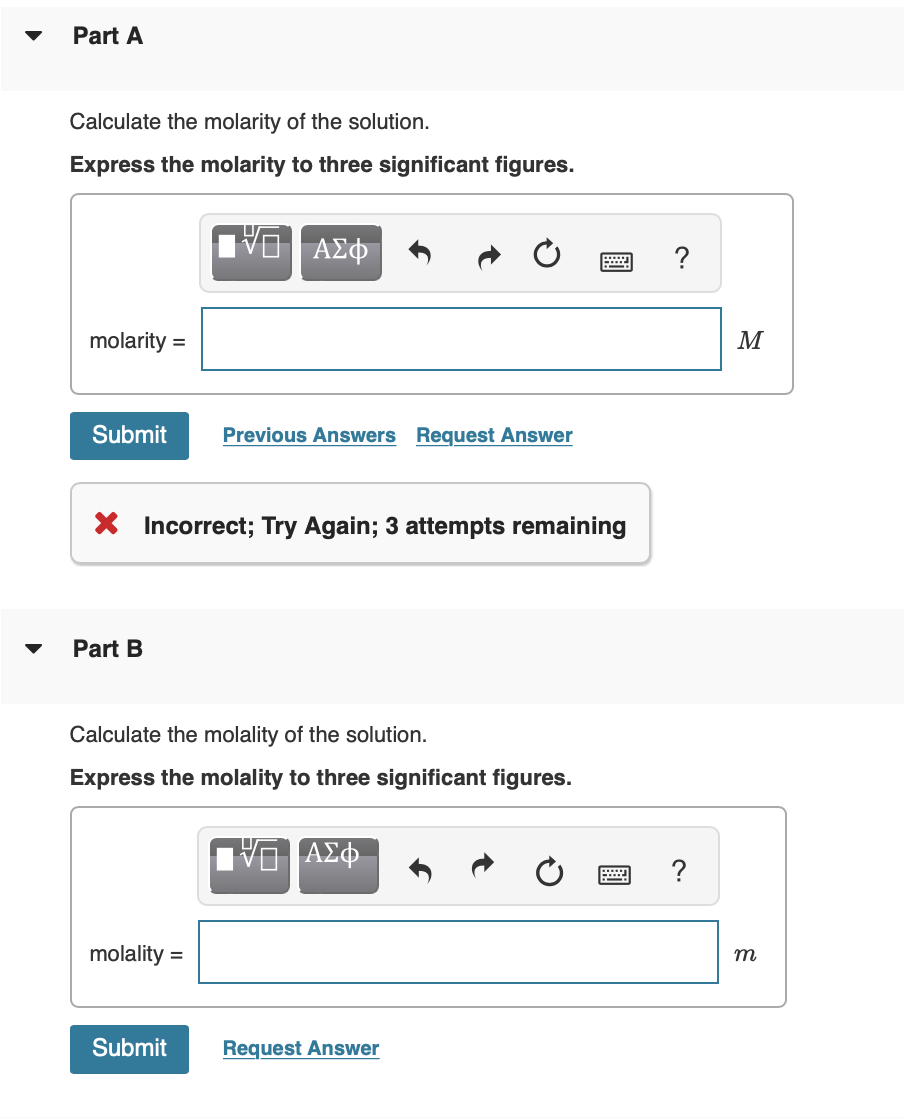
An aqueous solution is made using of diluted to a total solution volume of . (Assume a density of for the solution.)
Calculate the molarity of the solution. Express the molarity to three significant figures. Part B Calculate the molality of the solution. Express the molality to three significant figures.
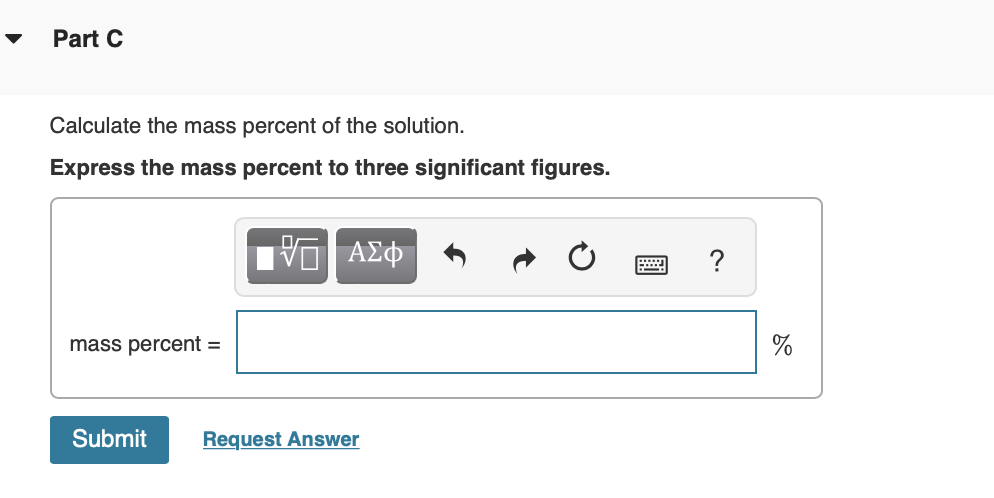
Calculate the mass percent of the solution. Express the mass percent to three significant figures.
Expert Answer
1) M ( molarity) number of moles in 1L of solution weight of solute 60.1g M =