Home /
Expert Answers /
Chemistry /
an-aqueous-cdi-solution-is-electrolyzed-under-1-bar-pressure-using-platinum-electrodes-a-wri-pa347
(Solved): An aqueous CdI solution is electrolyzed under 1 bar pressure using platinum electrodes. (a) Wri ...
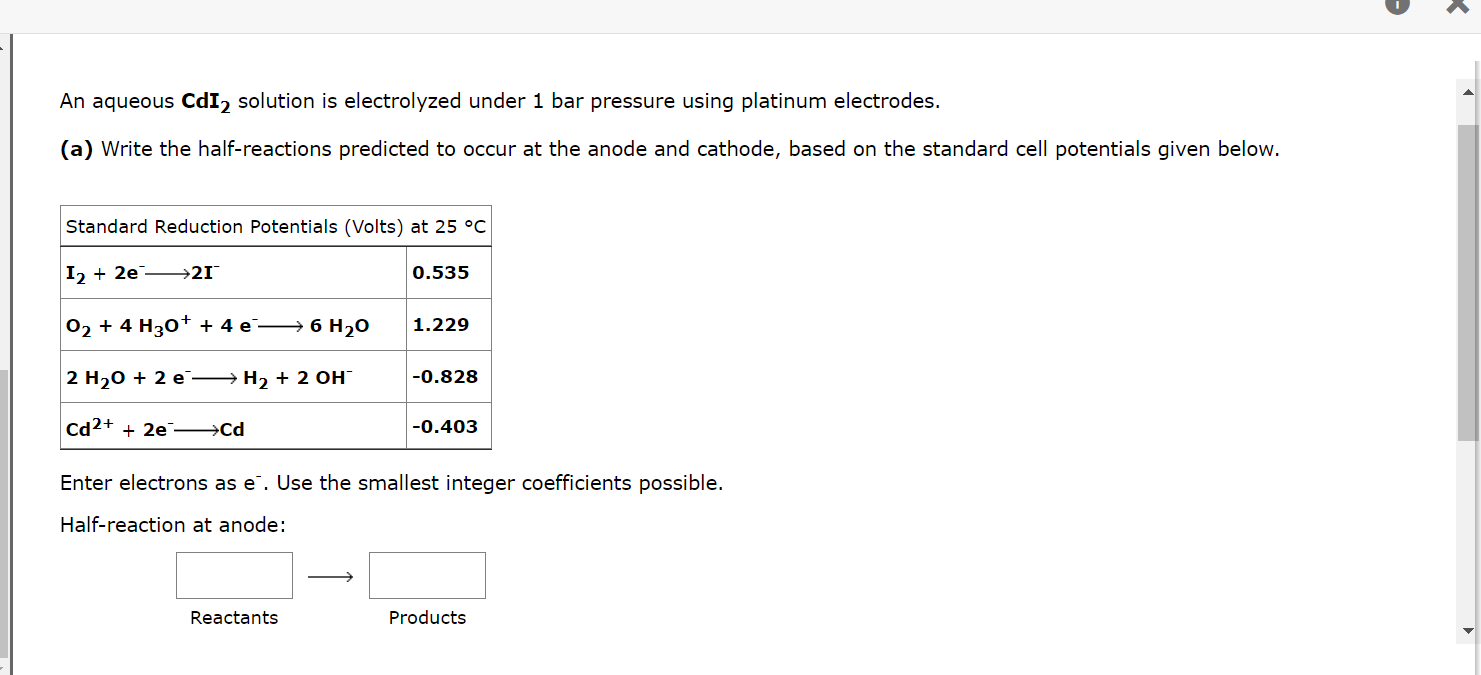
An aqueous CdI? solution is electrolyzed under 1 bar pressure using platinum electrodes. (a) Write the half-reactions predicted to occur at the anode and cathode, based on the standard cell potentials given below. Standard Reduction Potentials (Volts) at 25 °C I2 +2e ?21 O? + 4 H3O+ + 4 e?? 6 H?0 2 H?O + 2 e H? + 2 OH™ Cd2+ + 2e ??Cd 0.535 Reactants 1.229 -0.828 -0.403 Enter electrons as e. Use the smallest integer coefficients possible. Half-reaction at anode: Products K
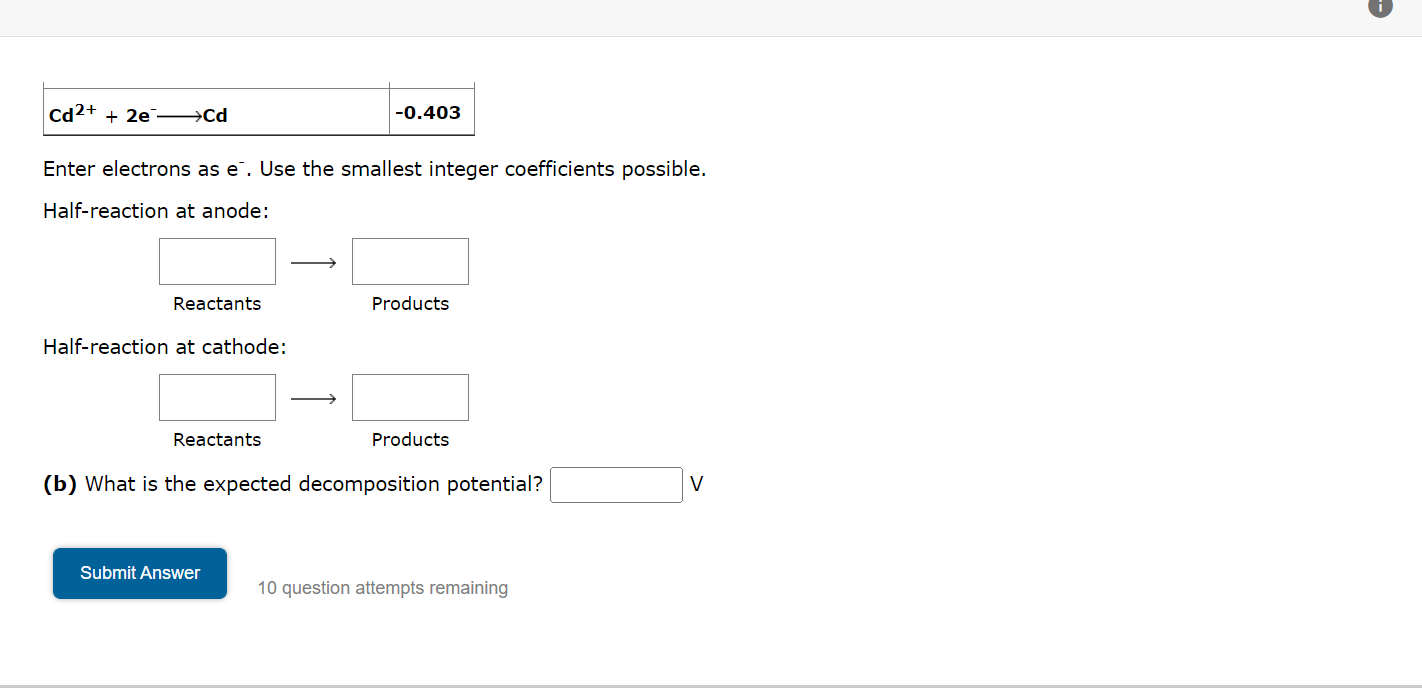
Cd2+ + 2e ??Cd Enter electrons as e. Use the smallest integer coefficients possible. Half-reaction at anode: Reactants Half-reaction at cathode: Reactants -0.403 Submit Answer Products Products (b) What is the expected decomposition potential? 10 question attempts remaining V
Expert Answer
a. Anode Half reaction at the anode: Cd2+ + 2e- = Cd 2 H2O + 2e- = H2 + 2 OH- Overall half reaction at anode: Cd2+ + 2 H2O + 4 e- = Cd + H2 + 2 OH- Cathode Half reaction at the cathode: 2I- = I2 + 2e- 6 H2O = O2 + 4 H3O + 4 e- Overall cathode: 6 H2O