Home /
Expert Answers /
Chemistry /
an-1-h-35-cl-has-a-force-constant-of-516-n-m-calculate-the-vibrational-frequency-of-the-molec-pa486
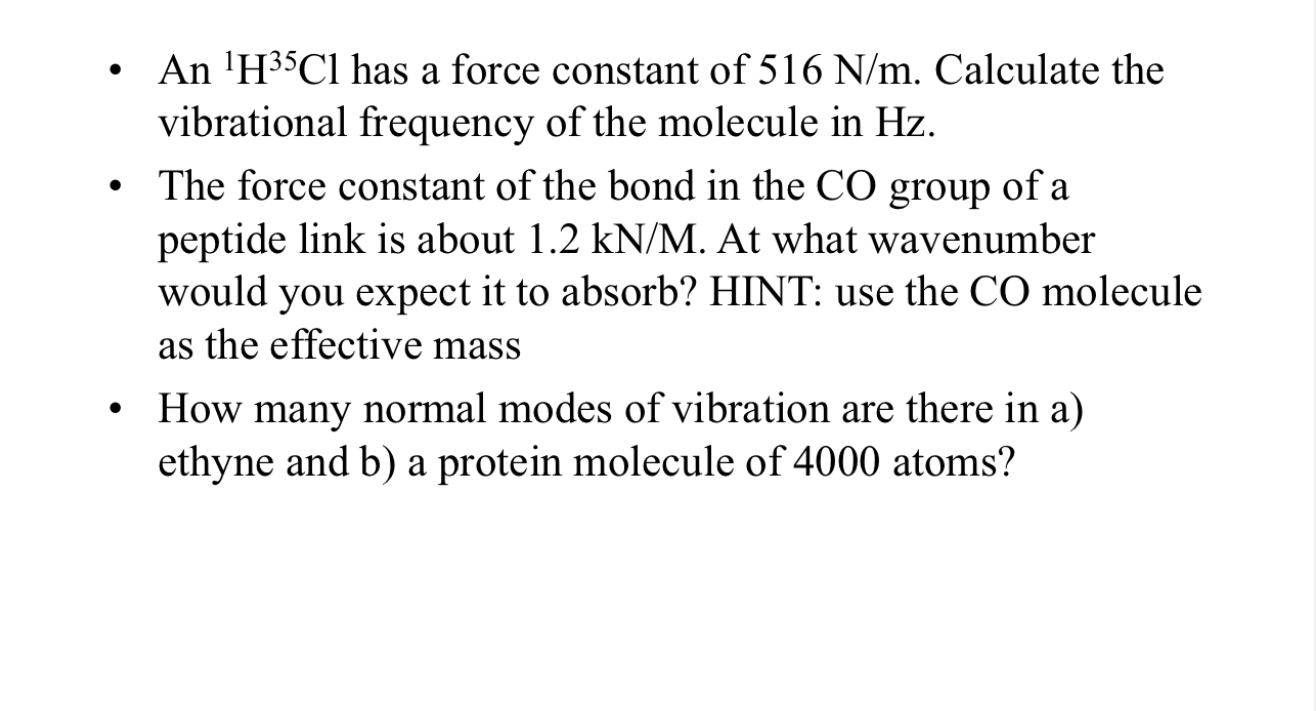
(Solved): An ^(1)H^(35)Cl has a force constant of 516(N)/(m). Calculate the vibrational frequency of the molec ...
An
^(1)H^(35)Clhas a force constant of
516(N)/(m). Calculate the vibrational frequency of the molecule in Hz . The force constant of the bond in the CO group of a peptide link is about
1.2k(N)/(M). At what wavenumber would you expect it to absorb? HINT: use the CO molecule as the effective mass How many normal modes of vibration are there in a) ethyne and b) a protein molecule of 4000 atoms?