Home /
Expert Answers /
Chemistry /
ammonia-mathrm-nh-3-mathrm-g-and-hydrogen-chloride-mathrm-hcl-mathrm-g-pa862
(Solved): Ammonia, \( \mathrm{NH}_{3}(\mathrm{~g}) \), and hydrogen chloride, \( \mathrm{HCl}(\mathrm{g}) \) ...
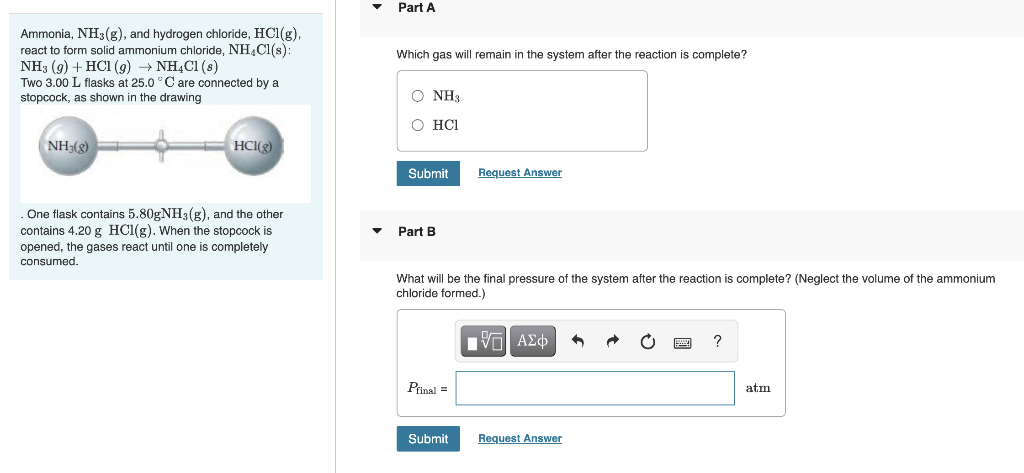
Ammonia, \( \mathrm{NH}_{3}(\mathrm{~g}) \), and hydrogen chloride, \( \mathrm{HCl}(\mathrm{g}) \), react to form solid ammonium chloride, \( \mathrm{NH}_{4} \mathrm{Cl}(\mathrm{s}) \) : \( \mathrm{NH}_{3}(g)+\mathrm{HCl}(g) \rightarrow \mathrm{NH}_{4} \mathrm{Cl}(s) \) Which gas will remain in the system after the reaction is complete? Two \( 3.00 \mathrm{~L} \) flasks at \( 25.0^{\circ} \mathrm{C} \) are connected by a stopcock, as shown in the drawing . One flask contains \( 5.80 \mathrm{gNH}_{3}(\mathrm{~g}) \), and the other contains \( 4.20 \mathrm{~g} \mathrm{HCl}(\mathrm{g}) \). When the stopcock is Part B opened, the gases react until one is completely consumed. What will be the final pressure of the system after the reaction is complete? (Neglect the volume of the ammonium chloride formed.)
What mass of ammonium chloride will be formed?
Expert Answer
The reaction between ammonia gas and hydrogen chloride gas forms ammonium chloride solid. As per the above-balanced chemical equation, the stoichiometric ratio of the number of moles of ammonia to the number of moles of hydrogen chloride is 1:1. Part