Home /
Expert Answers /
Chemistry /
all-is-correct-which-molecule-has-the-largest-bond-angle-in-each-series-a-sf2-b-tef2-pa597
(Solved): all is correct ? Which molecule has the largest bond angle in each series? A. SF2 B. TeF2 ...
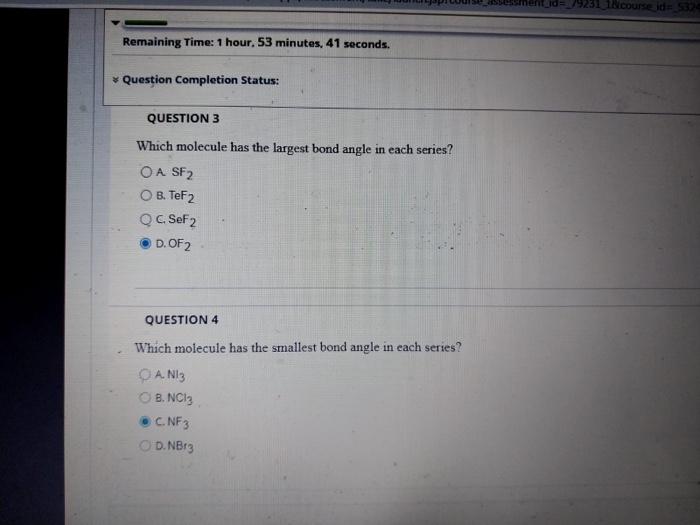 all is correct ? ????
all is correct ? ????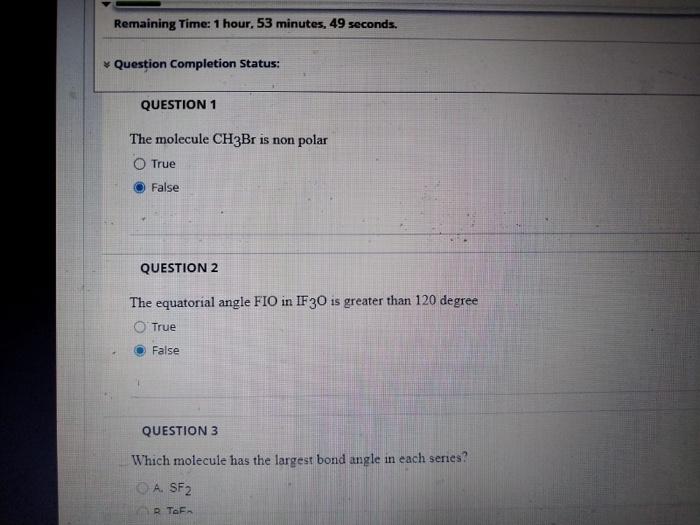
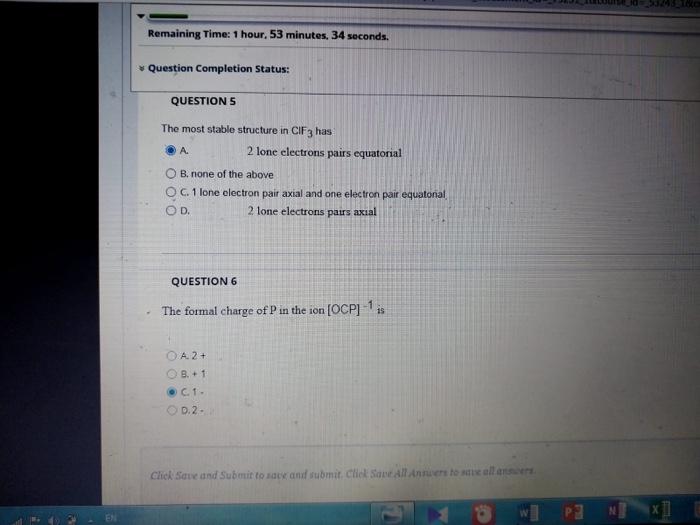
![The formal charge of \( P \) in the ion \( [O C P]^{-1} \) is
A. \( 2+ \)
B. +1
C. 1 -
D. 2 .
QUESTION 7
The angle \( \mathrm](https://media.cheggcdn.com/study/8a0/8a062c3d-4621-4b11-b7e8-1e222bf140a5/image)
Which molecule has the largest bond angle in each series? A. B. C. D. QUESTION 4 Which molecule has the smallest bond angle in each series? A. B. c. D. NBr3
The molecule is non polar True False QUESTION 2 The equatorial angle in is greater than 120 degree True False QUESTION 3 Which molecule has the largest bond angle in each series? A.
The most stable structure in has A. 2 lone electrons pairs equatorial B. none of the above C. 1 lone olectron pair axial and one electron pair equatonal D. 2 lone electrons pairs axial QUESTION 6 The formal charge of in the ion is A. C. 1 . p. 2.
The formal charge of in the ion is A. B. +1 C. 1 - D. 2 . QUESTION 7 The angle in the tetrahedral is smaller than 109 degree True False Crick Save and Submit to save ard thbmit. Chickeve. All ithaters to aque all answoers.
Expert Answer
Que 1) ans- molecule CH3Br is polar, not nonpolar.In CH3Br molecule, the carbon atom is sp3 hybridized, and the bond between carbon and bromine is po