Home /
Expert Answers /
Chemistry /
all-amino-acids-have-two-ionizable-functional-groups-an-alpha-amino-group-average-math-pa722
(Solved): All amino acids have two ionizable functional groups: an \( \alpha \)-amino group (average \( \math ...
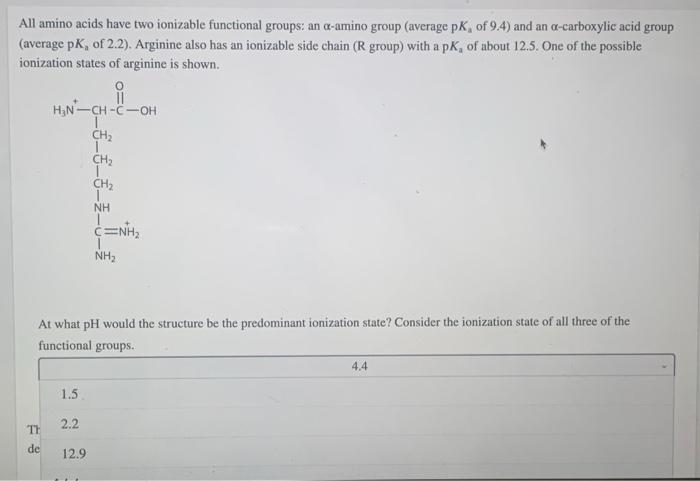

All amino acids have two ionizable functional groups: an \( \alpha \)-amino group (average \( \mathrm{p} K_{\mathrm{a}} \) of \( 9.4 \) ) and an \( \alpha \)-carboxylic acid group (average \( \mathrm{p}_{\mathrm{a}} \) of 2.2). Arginine also has an ionizable side chain ( \( \mathrm{R} \) group) with a \( \mathrm{p} K_{\mathrm{a}} \) of about 12.5. One of the possible ionization states of arginine is shown. At what \( \mathrm{pH} \) would the structure be the predominant ionization state? Consider the ionization state of all three of the functional groups.
At what \( \mathrm{pH} \) would the structure be the predominant ionization state? Consider the ionization state of all three of the functional groups.
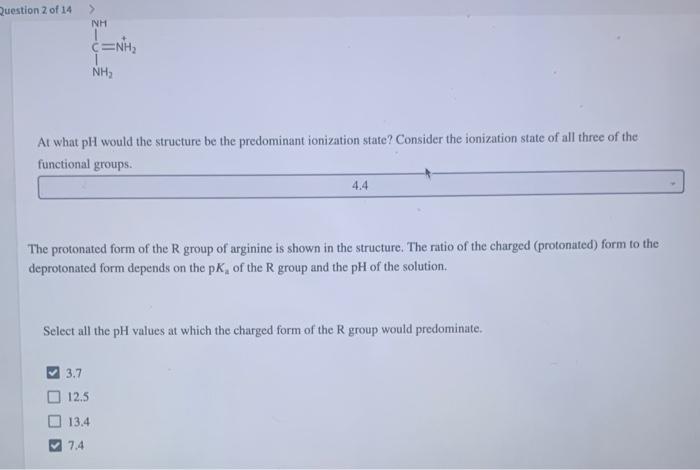
At what pH would the structure be the predominant ionization state? Consider the ionization state of all three of the The protonated form of the \( \mathrm{R} \) group of arginine is shown in the structure. The ratio of the charged (protonated) form to the deprotonated form depends on the \( \mathrm{p}_{\mathrm{a}} \) of the \( \mathrm{R} \) group and the \( \mathrm{pH} \) of the solution. Select all the \( \mathrm{pH} \) values at which the charged form of the \( \mathrm{R} \) group would predominate. \( 3.7 \) \( 12.5 \) \( 13.4 \) \( 7.4 \)