Home /
Expert Answers /
Chemistry /
acids-bases-handout-name-date-1-fill-in-the-table-below-2-write-the-formula-of-the-conjugate-pa788
(Solved): Acids/Bases Handout Name Date: 1. Fill in the table below. 2. Write the formula of the conjugate ...
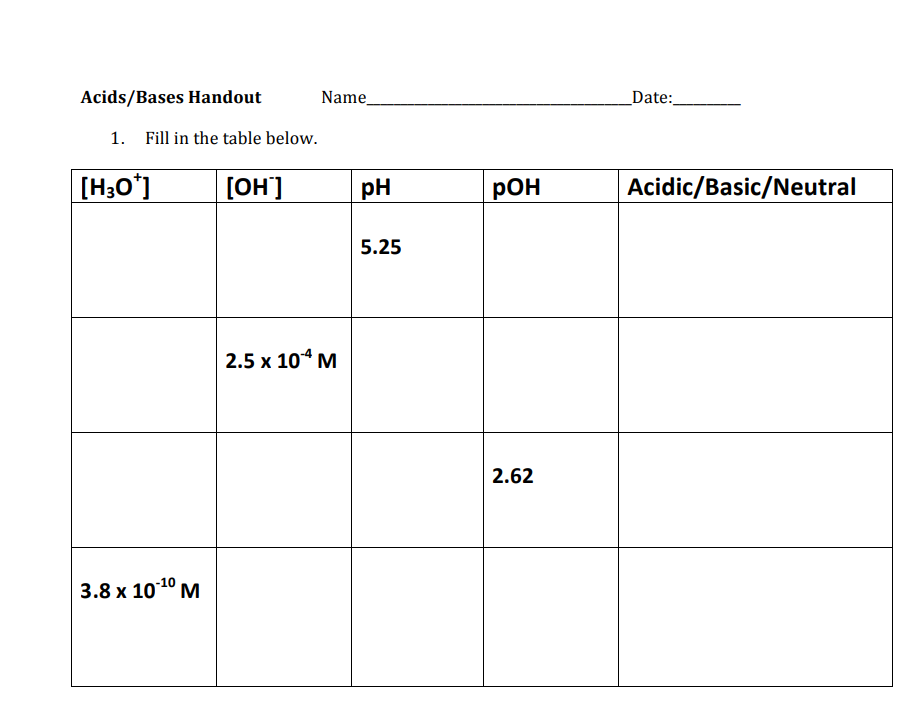
Acids/Bases Handout Name Date: 1. Fill in the table below.
2. Write the formula of the conjugate acid of the following. a. \( \mathrm{OH}^{-} \) b. \( \mathrm{NH}_{2}^{-} \) c. \( \mathrm{H}_{2} \mathrm{PO}_{4}^{-} \) 3. Write the formula of the conjugate base of the following. a. \( \mathrm{H}_{2} \mathrm{PO}_{4}^{-} \) b. \( \mathrm{HC}_{4} \mathrm{H}_{7} \mathrm{O}_{2} \) c. \( \mathrm{HCO}_{3} \) 4. Write a balanced equation for each of the following neutralization reactions. a. \( \mathrm{H}_{2} \mathrm{SO}_{4} \) and \( \mathrm{RbOH} \) b. \( \mathrm{Ca}(\mathrm{OH})_{2} \) and \( \mathrm{HClO}_{4} \) c. \( \mathrm{H}_{3} \mathrm{PO}_{4} \) and \( \mathrm{Mg}(\mathrm{OH})_{2} \)