Home /
Expert Answers /
Chemistry /
acid-base-practice-test-acids-taste-a-sweet-b-sour-2-acids-make-litmus-paper-tum-a-red-b-pa611
(Solved): Acid Base Practice Test Acids taste a. sweet. b. sour. 2. Acids make litmus paper tum a. red. b ...
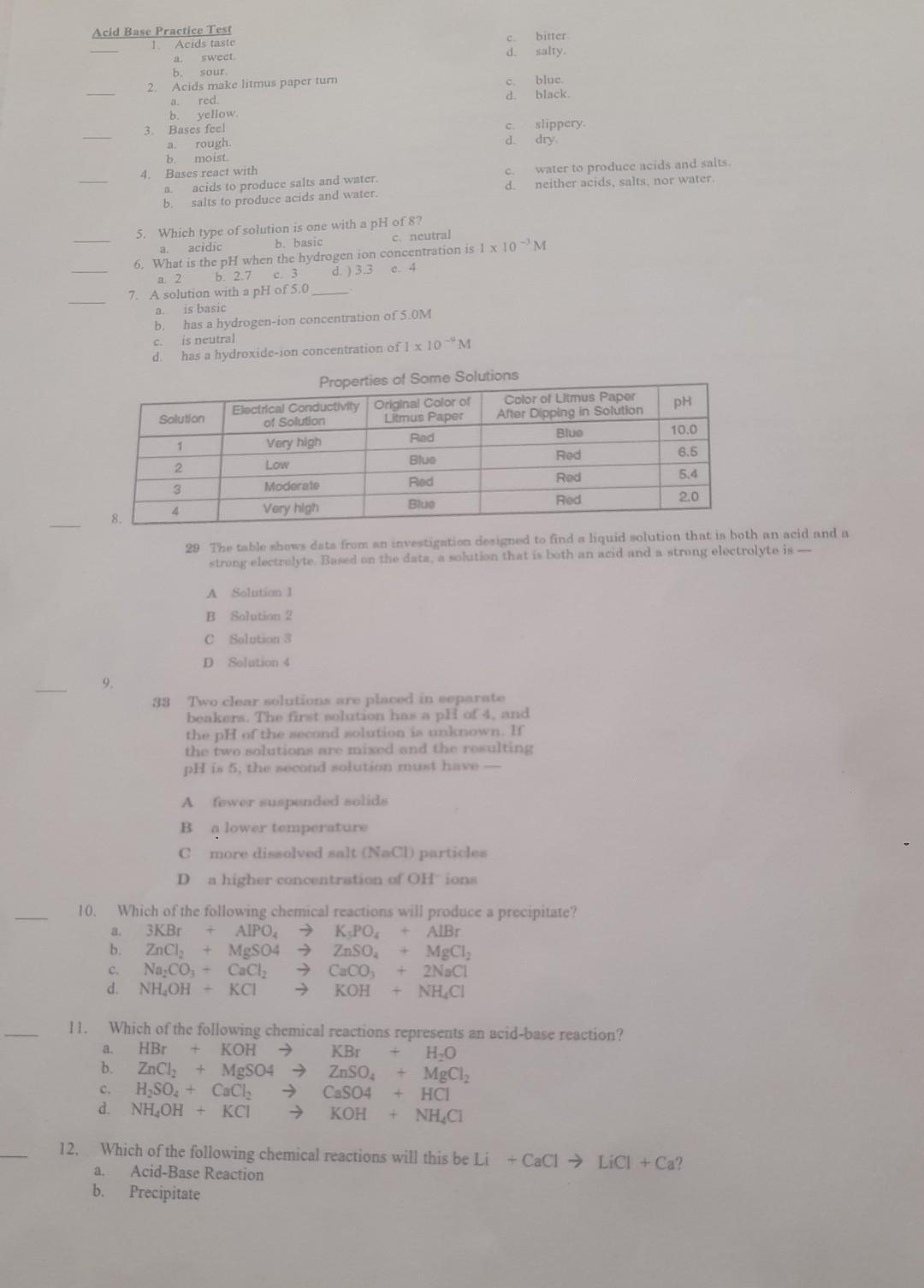
Acid Base Practice Test Acids taste a. sweet. b. sour. 2. Acids make litmus paper tum a. red. b. yellow. 3. Bases feel a. rough. b. moist. 4. Bases react with a. acids to produce salts and water. b. salts to produce acids and water. c. bitter d. salty. c. blue. d. black. c. slippery. d. dry. c. water to produce acids and salts. d. neither acids, salts, nor water. 5. Which type of solution is one with a pH of 8 ? a. acidic b. basic c. neutral 6. What is the \\( \\mathrm{pH} \\) when the hydrogen ion concentration is \\( 1 \\times 10^{-3} \\mathrm{M} \\) a. 2 b. 2.7 c. 3 d. ) 3.3 c. 4 7. A solution with a \\( \\mathrm{pH} \\) of 5.0 a. is basic b. has a hydrogen-ion concentration of \\( 5.0 \\mathrm{M} \\) c. is neutral d. has a hydroxide-ion concentration of \\( 1 \\times 10^{-4} \\mathrm{M} \\) Properties of Some Solutions \\begin{tabular}{|c|c|c|c|c|} \\hline Solution & \\( \\begin{array}{c}\\text { Eloctrical Conductivity } \\\\ \\text { of Solutlon }\\end{array} \\) & \\( \\begin{array}{c}\\text { Original Color of } \\\\ \\text { Litmus Paper }\\end{array} \\) & \\( \\begin{array}{c}\\text { Color of Litmus Paper } \\\\ \\text { After Dipping in Solution }\\end{array} \\) & \\( \\mathrm{pH} \\) \\\\ \\hline 1 & Very high & Rad & Blue & 10.0 \\\\ \\hline 2 & Low & Blue & Red & 6.5 \\\\ \\hline 3 & Moderate & Rod & Red & 5.4 \\\\ \\hline 4 & Very high & Bluo & Red & 2.0 \\\\ \\hline \\end{tabular} 29 The cable shows data from an investigntion designed to find a liquid solution that is both an acid and a strong electralyte. Based on the data, a solution that is both an acid and a strong electrolyte is - A Solution 1 B Solvtion 2 C Solution 3 D Solution 4 9. 33 Two clear solutions are placed in separate beakers. The first molixtion has a pli of 4, and the \\( \\mathrm{pH} \\) of the second nolution is unknown. If the two solutions are mixed and the resulting \\( \\mathrm{pH} \\) is 5 , the second solution munt hawe - A fewer suspended solides B ? lower temperature C more dissolved salt \\( (\\mathrm{NaCl}) \\) particles D a higher concentration of \\( \\mathrm{OH}^{-} \\)ions 10. Which of the following chemical reactions will produce a precipitate? a. \\( 3 \\mathrm{KBr}+\\mathrm{AlPO}_{4} \\rightarrow \\mathrm{K}_{3} \\mathrm{PO}_{4}+\\mathrm{AlBr} \\) b. \\( \\mathrm{ZnCl}_{2}+\\mathrm{MgSO}_{4} \\rightarrow \\mathrm{ZnSO}_{4}+\\mathrm{MgCl}_{2} \\) c. \\( \\mathrm{Na}_{2} \\mathrm{CO}_{3}+\\mathrm{CaCl}_{2} \\rightarrow \\mathrm{CaCO}_{3}+2 \\mathrm{NaCl} \\) d. \\( \\mathrm{NH}_{4} \\mathrm{OH}+\\mathrm{KCl} \\rightarrow \\mathrm{KOH}+\\mathrm{NH}_{4} \\mathrm{Cl} \\) 11. Which of the following chemical reactions represents an acid-base reaction? a. \\( \\mathrm{HBr}+\\mathrm{KOH} \\rightarrow \\mathrm{KBr}+\\mathrm{H}_{2} \\mathrm{O} \\) b. \\( \\mathrm{ZnCl}_{2}+\\mathrm{MgSO} 4 \\rightarrow \\mathrm{ZnSO}_{4}+\\mathrm{MgCl}_{2} \\) c. \\( \\mathrm{H}_{2} \\mathrm{SO}_{4}+\\mathrm{CaCl}_{2} \\rightarrow \\mathrm{CaSO}_{4}+\\mathrm{HCl} \\) d. \\( \\mathrm{NH}_{4} \\mathrm{OH}+\\mathrm{KCl} \\rightarrow \\mathrm{KOH}+\\mathrm{NH}_{4} \\mathrm{Cl} \\) 12. Which of the following chemical reactions will this be \\( \\mathrm{Li}+\\mathrm{CaCl} \\rightarrow \\mathrm{LiCl}+\\mathrm{Ca} \\) ? a. Acid-Base Reaction b. Precipitate