Home /
Expert Answers /
Chemistry /
according-to-the-ideal-gas-law-a-10-02mol-sample-of-nitrogen-gas-in-a-0-8016l-container-at-501-pa323
(Solved): According to the ideal gas law, a 10.02mol sample of nitrogen gas in a 0.8016L container at 501. ...
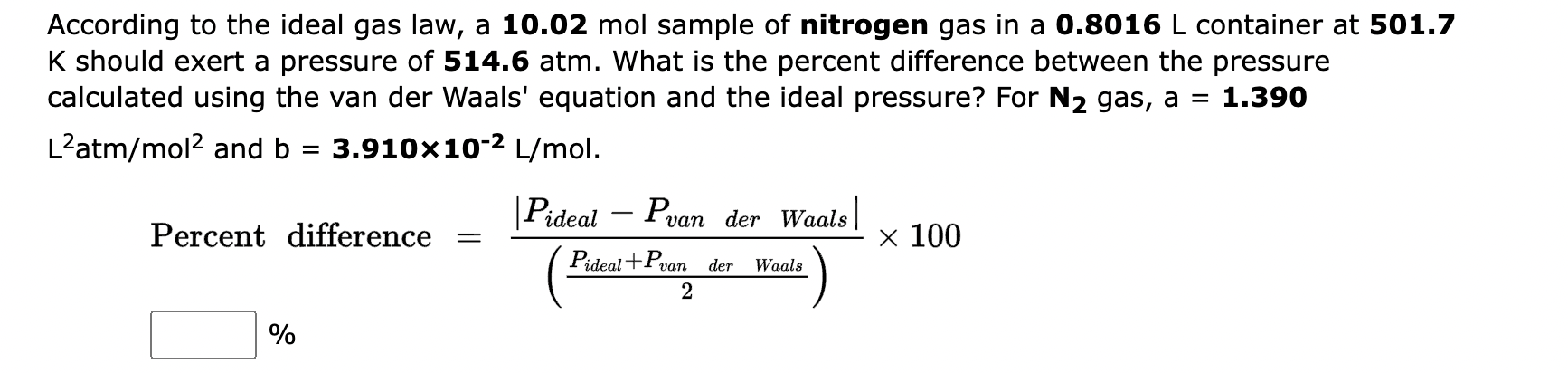
According to the ideal gas law, a sample of nitrogen gas in a container at should exert a pressure of . What is the percent difference between the pressure calculated using the van der Waals' equation and the ideal pressure? For gas,
Expert Answer
Given :----> Number of moles (n) = 10.02 mol---> Volume (V) = 0.8016 L---> Temperature = 501.7 K---> Ideal pressure (Pideal) = 514.6 atm---> value of