Home /
Expert Answers /
Chemistry /
according-to-the-following-reaction-how-many-moles-of-hydrobromic-acid-are-necessary-to-form-0-799-pa733
(Solved): According to the following reaction, how many moles of hydrobromic acid are necessary to form 0.799 ...
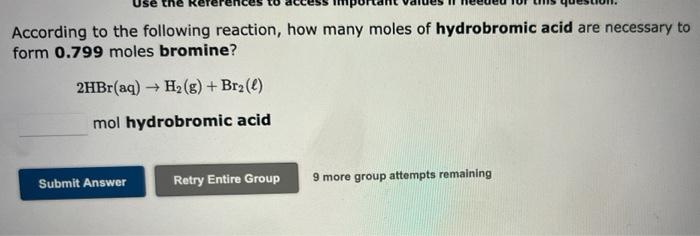
According to the following reaction, how many moles of hydrobromic acid are necessary to form 0.799 moles bromine? 2HBr(aq) ? H?(g) + Br?(l) mol hydrobromic acid Retry Entire Group 9 more group attempts remaining Submit Answer
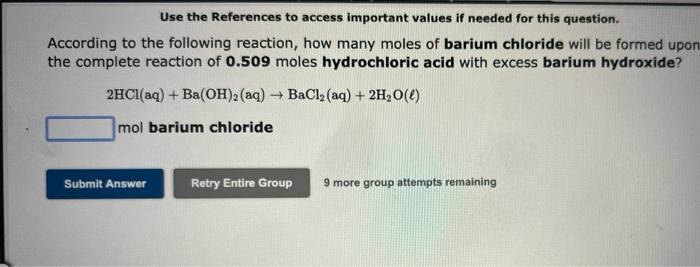
Use the References to access important values if needed for this question. According to the following reaction, how many moles of barium chloride will be formed upon the complete reaction of 0.509 moles hydrochloric acid with excess barium hydroxide? 2HCl(aq) + Ba(OH)2 (aq) ? BaCl? (aq) + 2H?O(l) mol barium chloride 9 more group attempts remaining Submit Answer Retry Entire Group
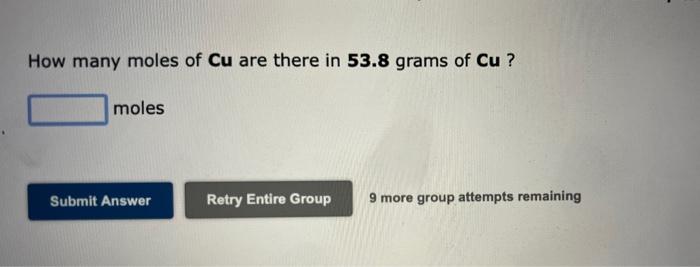
How many moles of Cu are there in 53.8 grams of Cu ? moles Submit Answer Retry Entire Group 9 more group attempts remaining
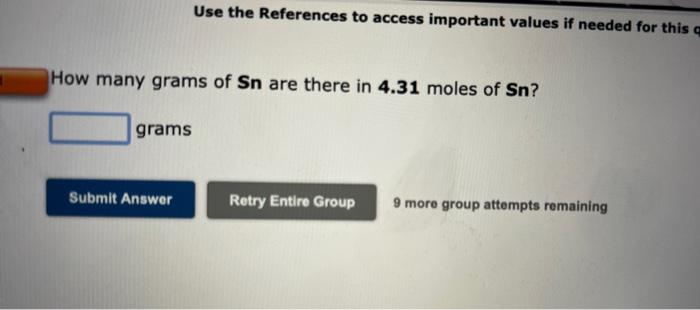
Use the References to access important values if needed for this q How many grams of Sn are there in 4.31 moles of Sn? grams Submit Answer Retry Entire Group 9 more group attempts remaining