Home /
Expert Answers /
Chemistry /
a14-the-equilibrium-constant-for-the-decomposition-of-sulfur-trioxide-reaction-shown-below-is-2-pa684
(Solved): A14. The equilibrium constant for the decomposition of sulfur trioxide (reaction shown below) is 2 ...
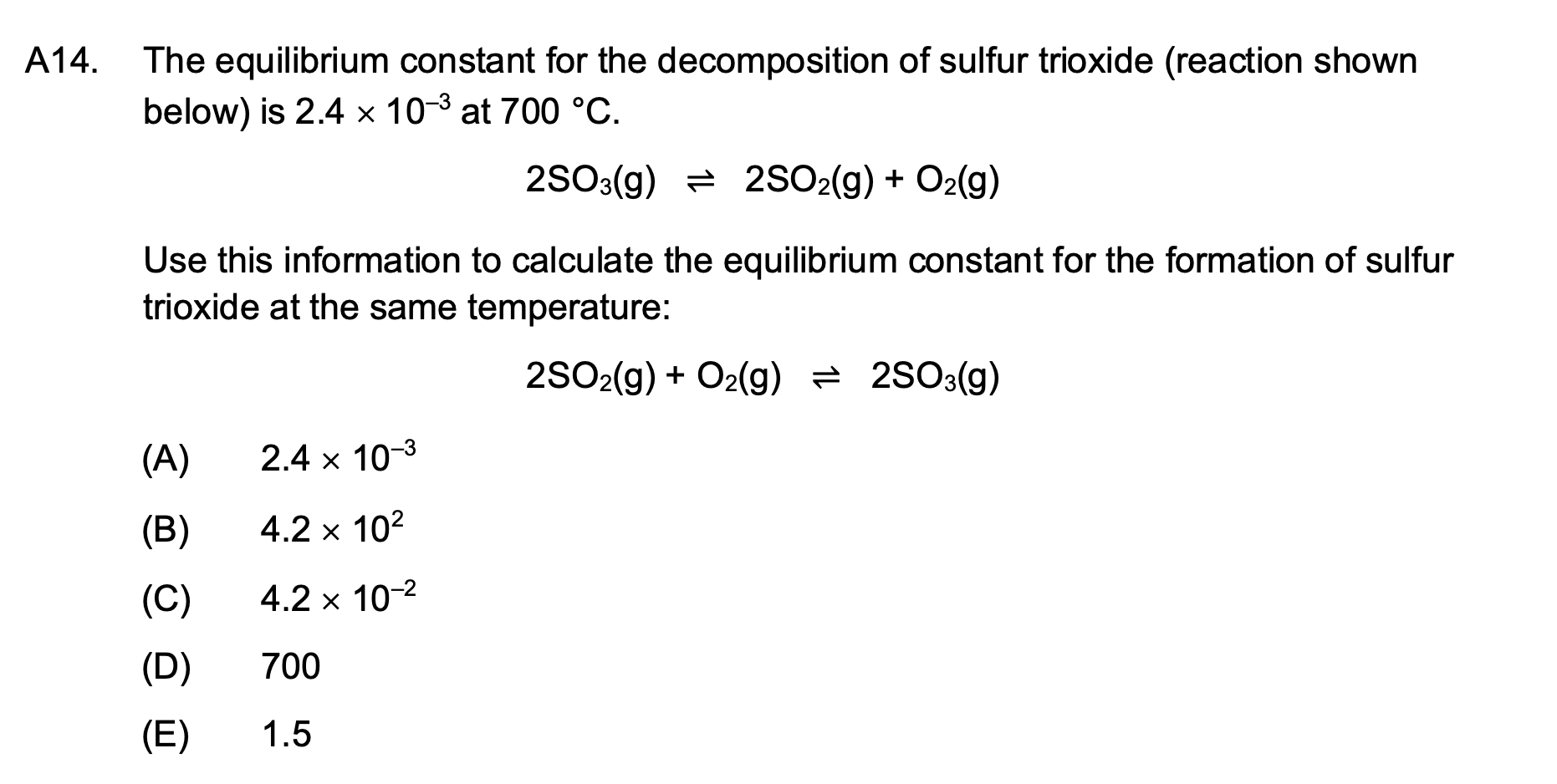
A14. The equilibrium constant for the decomposition of sulfur trioxide (reaction shown below) is 2.4 x 10-³ at 700 °C. 2SO3(g) 2SO2(g) + O?(g) Use this information to calculate the equilibrium constant for the formation of sulfur trioxide at the same temperature: 2SO2(g) + O2(g) = 2SO3(g) (A) 2.4 x 10-³ (B) 4.2 x 10² (C) 4.2 x 10-² (D) 700 (E) 1.5