Home /
Expert Answers /
Chemistry /
a-what-is-the-hybridization-of-the-central-atom-in-nobr-hybridization-what-are-the-approx-pa365
(Solved): A. What is the hybridization of the central atom in NOBr? Hybridization \( = \) What are the approx ...
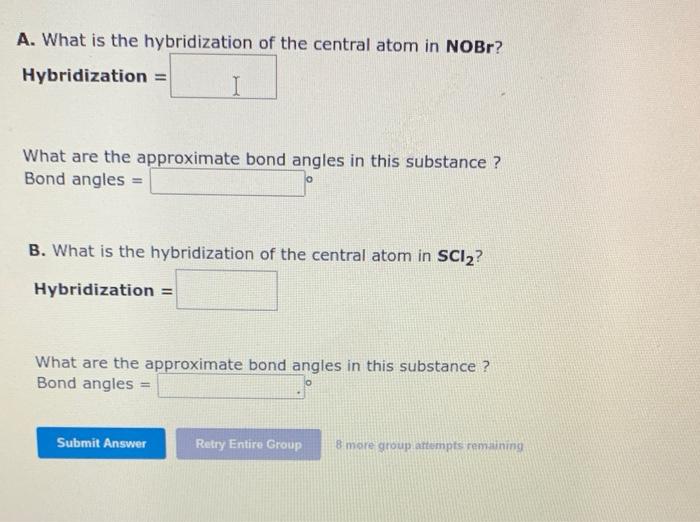
A. What is the hybridization of the central atom in NOBr? Hybridization \( = \) What are the approximate bond angles in this substance? Bond angles \( = \) B. What is the hybridization of the central atom in \( \mathbf{S C l}_{2} \) ? Hybridization \( = \) What are the approximate bond angles in this substance? Bond angles \( = \) 8 mare group attempts remainime?
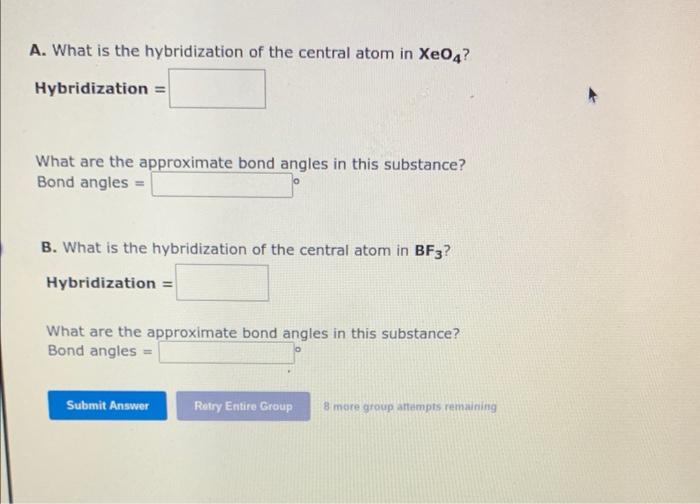
A. What is the hybridization of the central atom in \( \mathrm{XeO}_{4} \) ? Hybridization \( = \) What are the approximate bond angles in this substance? Bond angles \( = \) B. What is the hybridization of the central atom in \( \mathbf{B F}_{3} \) ? Hybridization \( = \) What are the approximate bond angles in this substance? Bond angles =
Expert Answer
PART 1 (A) The Hybdization of NOBr is sp² The bond angl