Home /
Expert Answers /
Chemistry /
a-voltaic-electrochemical-cell-is-constructed-using-the-following-reaction-the-half-cell-component-pa696
(Solved): A voltaic electrochemical cell is constructed using the following reaction. The half-cell component ...
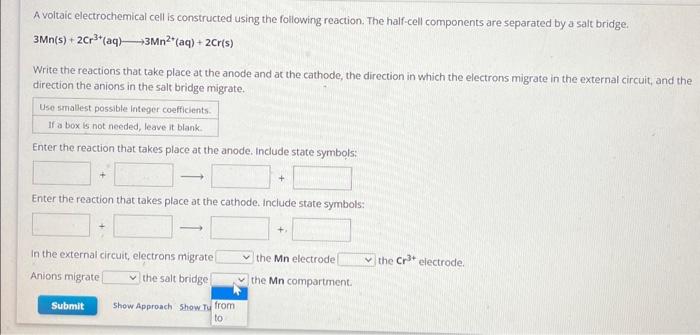
A voltaic electrochemical cell is constructed using the following reaction. The half-cell components are separated by a salt bridge. Write the reactions that take place at the anode and at the cathode, the direction in which the electrons migrate in the external circuit, and the direction the anions in the salt bridge migrate. Enter the reaction that takes place at the anode. include state symbols: Enter the reaction that takes place at the cathode. Include state symbols: In the external circuit, electrons migrate the Mn electrode electrode. Anions migrate the salt bridge the Mn compartment.