Home /
Expert Answers /
Chemistry /
a-using-the-table-below-what-would-the-ionic-strength-of-seawater-be-if-only-magnesium-and-chlorin-pa519
(Solved): (a) Using the table below what would the ionic strength of seawater be if only magnesium and chlorin ...
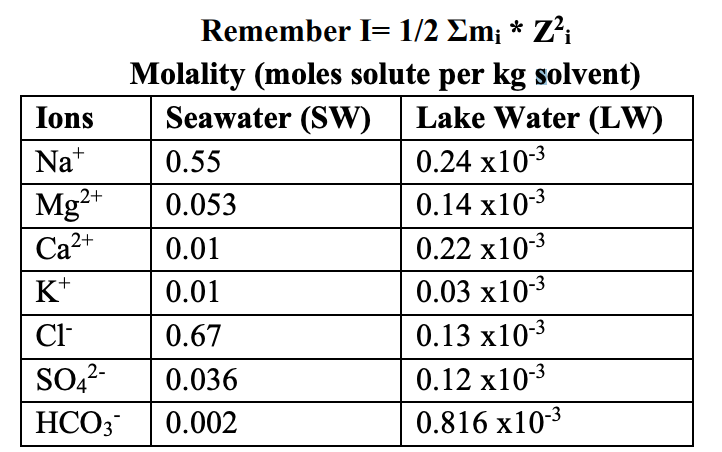
(a) Using the table below what would the ionic strength of seawater be if only magnesium and chlorine were in this solution? [mol kg -1 ] (b) How does this change if we include all the ions present in seawater shown in the table?
Remember \( I=1 / 2 \Sigma m_{i} * Z_{i}^{2} \) Molality (moles solute per kg solvent)
Expert Answer
a)Following the formula, we have m for mola