Home /
Expert Answers /
Chemistry /
a-use-the-periodic-table-to-write-the-formula-including-the-charge-for-the-simple-ion-formed-by-pa399
(Solved): a. Use the periodic table to write the formula (Including the charge) for the simple ion formed by ...
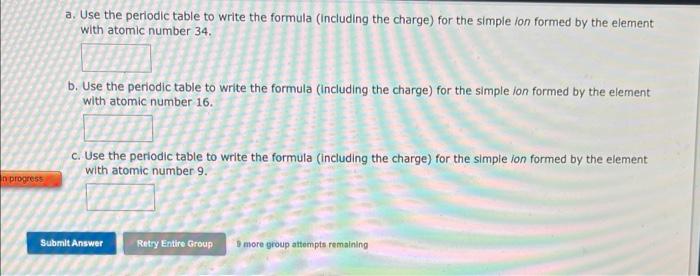
a. Use the periodic table to write the formula (Including the charge) for the simple ion formed by the element with atomic number \( 34 . \) b. Use the periodic table to write the formula (Including the charge) for the simple ion formed by the element with atomic number \( 16 . \) c. Use the periodic table to write the formula (including the charge) for the simpie fon formed by the element with atomic number \( 9 . \) D more group attempts remaining
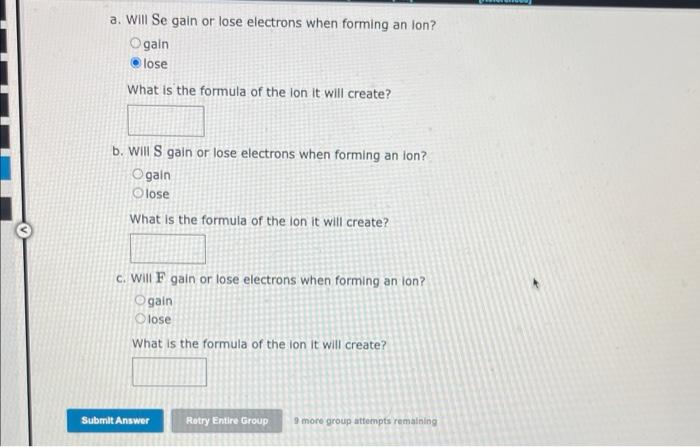
a. Will Se gain or lose electrons when forming an Ion? gain lose What is the formula of the ion it will create? b. Will \( \mathrm{S} \) gain or lose electrons when forming an ion? gain lose What is the formula of the ion it will create? c. Will F gain or lose electrons when forming an lon? gain lose What is the formula of the ion it will create?
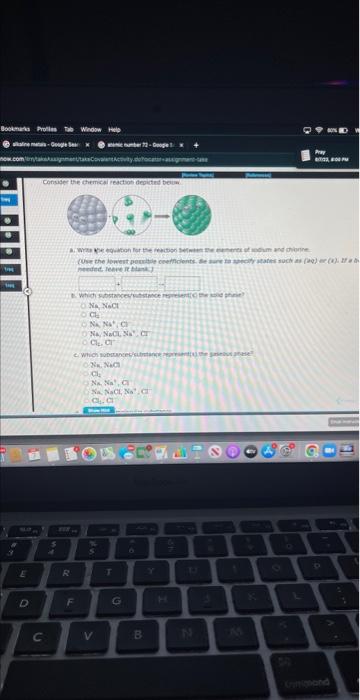
tole warer ? Nin \( \mathbb{N}^{+} \), \( \mathrm{C}^{4} \) \( \mathrm{B}_{\text {\&e }} \) ? ? Nan than 0 Nia. NaCH, \( \mathrm{Na}^{4}-\mathrm{C} \) - \( \mathrm{B}_{4}+\mathrm{CH} \)
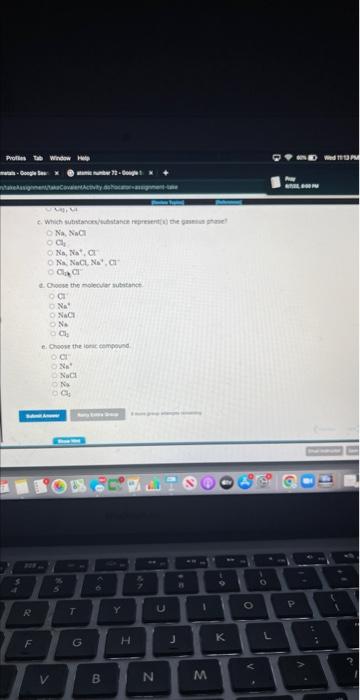
Nay siami \( \mathrm{CH}_{1} \) \( \mathrm{Na}, \mathrm{Na}^{+}, \mathrm{Cl} \) Nis. NaC1, \( \mathrm{Ne}^{4}+\mathrm{Cl}^{-} \) \( \mathrm{B}_{\mathrm{i}} \mathrm{CH}^{-1} \)