Home /
Expert Answers /
Physics /
a-system-has-3-energy-levels-with-energies-as-follows-state-1-8-mathrm-ev-state-2-pa123
(Solved): A system has 3 energy levels, with energies as follows: - State 1: \( 8 \mathrm{eV} \) - State 2: ...
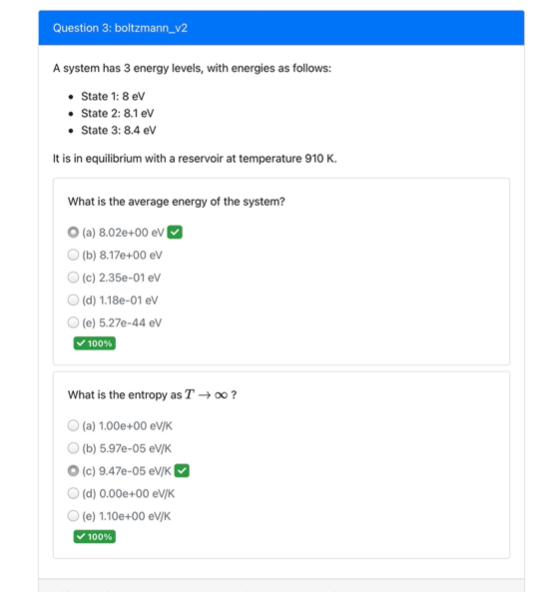
A system has 3 energy levels, with energies as follows: - State 1: \( 8 \mathrm{eV} \) - State 2: 8.1 eV - State 3: \( 8.4 \mathrm{eV} \) It is in equilibrium with a reservoir at temperature \( 910 \mathrm{~K} \). What is the average energy of the system? (a) \( 8.02 \mathrm{e}+00 \mathrm{eV} \) (b) \( 8.17 \mathrm{e}+00 \mathrm{eV} \) (c) 2.35e-01 eV (d) \( 1.18 \mathrm{e}-01 \mathrm{eV} \) (e) \( 5.27 \mathrm{e}-44 \mathrm{eV} \) What is the entropy as \( T \rightarrow \infty \) ? (a) \( 1.00 \mathrm{e}+00 \mathrm{eV} / \mathrm{K} \) (b) 5.97e-05 eV/K (c) 9.47e-05 eV/K (d) \( 0.00 \mathrm{e}+00 \mathrm{eV} / \mathrm{K} \) (e) \( 1.10 \mathrm{e}+00 \mathrm{eV} / \mathrm{K} \)
Expert Answer
given that , E1=8eV,E2=8.1eV,E3=8.4eV T=910K the average energy of the system is