Home /
Expert Answers /
Chemistry /
a-suggested-mechanism-for-the-gas-phase-decomposition-of-nitrous-oxide-is-step-1-slow-n2on2-pa996
(Solved): A suggested mechanism for the gas phase decomposition of nitrous oxide is: step 1 slow: N2ON2 ...
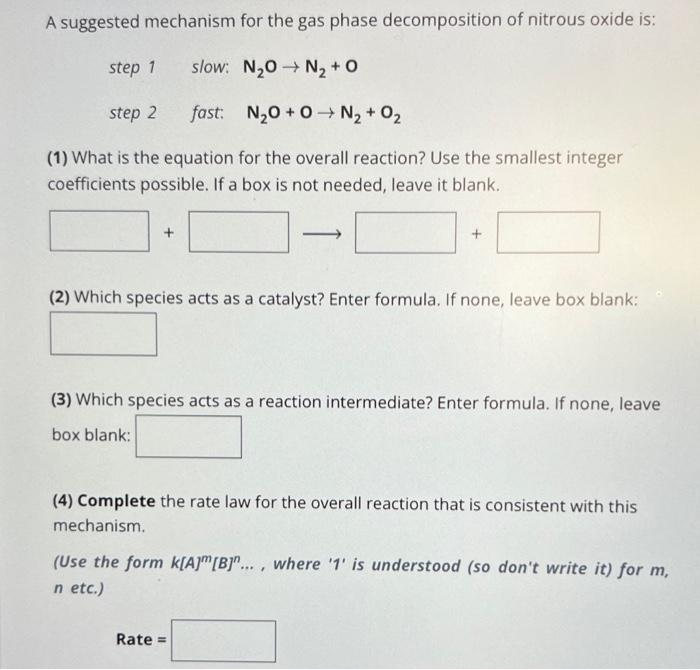
A suggested mechanism for the gas phase decomposition of nitrous oxide is: step 1 slow: step 2 fast: (1) What is the equation for the overall reaction? Use the smallest integer coefficients possible. If a box is not needed, leave it blank. (2) Which species acts as a catalyst? Enter formula. If none, leave box blank: (3) Which species acts as a reaction intermediate? Enter formula. If none, leave box blank: (4) Complete the rate law for the overall reaction that is consistent with this mechanism. (Use the form , where ' 1 ' is understood (so don't write it) for , etc.)
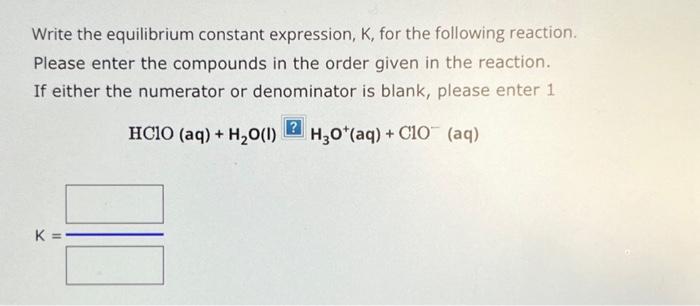
Write the equilibrium constant expression, , for the following reaction. Please enter the compounds in the order given in the reaction. If either the numerator or denominator is blank, please enter 1