Home /
Expert Answers /
Chemistry /
a-student-proposed-the-following-mechanism-for-the-gas-phase-reaction-of-fluorine-with-chlorine-dio-pa425
(Solved): A student proposed the following mechanism for the gas phase reaction of fluorine with chlorine dio ...

A student proposed the following mechanism for the gas phase reaction of fluorine with chlorine dioxide. step 1 fast: \( 2 \mathrm{ClO}_{2} \rightleftharpoons \mathrm{Cl}_{2} \mathrm{O}_{4} \) step 2 slow: \( \mathrm{Cl}_{2} \mathrm{O}_{4}+\mathrm{F}_{2} \longrightarrow 2 \mathrm{FClO}_{2} \) (1) What is the equation for the overall reaction? Use the smallest integer coefficients possible. If a box is not needed, leave it blank. (2) Enter the formula of any species that acts as a reaction intermediate? If none leave box blank: (3) Complete the rate law for the overall reaction that is consistent with this mechanism. Use the form \( \mathbf{k}[\mathbf{A}]^{\mathrm{m}}[\mathbf{B}]^{\mathrm{n}} \ldots \), where ' 1 ' is understood (so don't write it if it's a '1') for \( m, n \) etc.
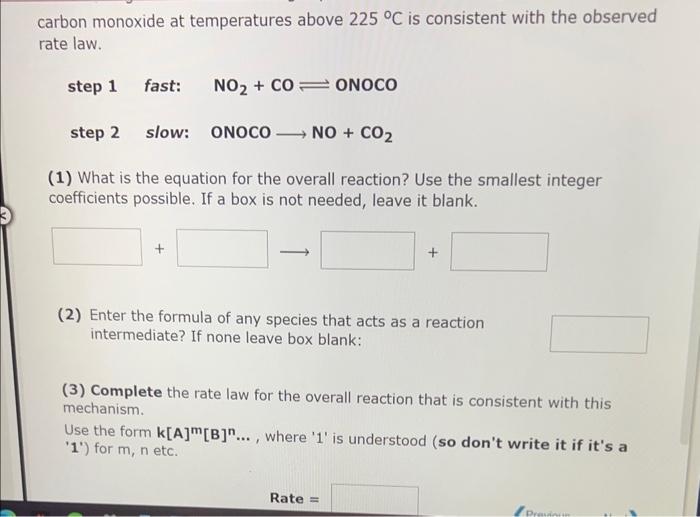
carbon monoxide at temperatures above \( 225^{\circ} \mathrm{C} \) is consistent with the observed rate law. step 1 fast: \( \mathrm{NO}_{2}+\mathrm{CO} \rightleftharpoons \mathrm{ONOCO} \) step 2 slow: \( \quad \) ONOCO \( \longrightarrow \mathrm{NO}+\mathrm{CO}_{2} \) (1) What is the equation for the overall reaction? Use the smallest integer coefficients possible. If a box is not needed, leave it blank. (2) Enter the formula of any species that acts as a reaction intermediate? If none leave box blank: (3) Complete the rate law for the overall reaction that is consistent with this mechanism. Use the form \( \mathbf{k}[\mathbf{A}]^{\mathrm{m}}[\mathrm{B}]^{\mathrm{n}} \ldots \), where ' 1 ' is understood (so don't write it if it's a ' 1 ') for \( m, n \) etc.