Home /
Expert Answers /
Chemistry /
a-student-dissolves-11-2-g-of-potassium-chloride-kcl-in-300-g-of-water-in-a-well-insulated-open-pa483
(Solved): A student dissolves 11.2 g of potassium chloride (KCl) in 300. g of water in a well-insulated open ...
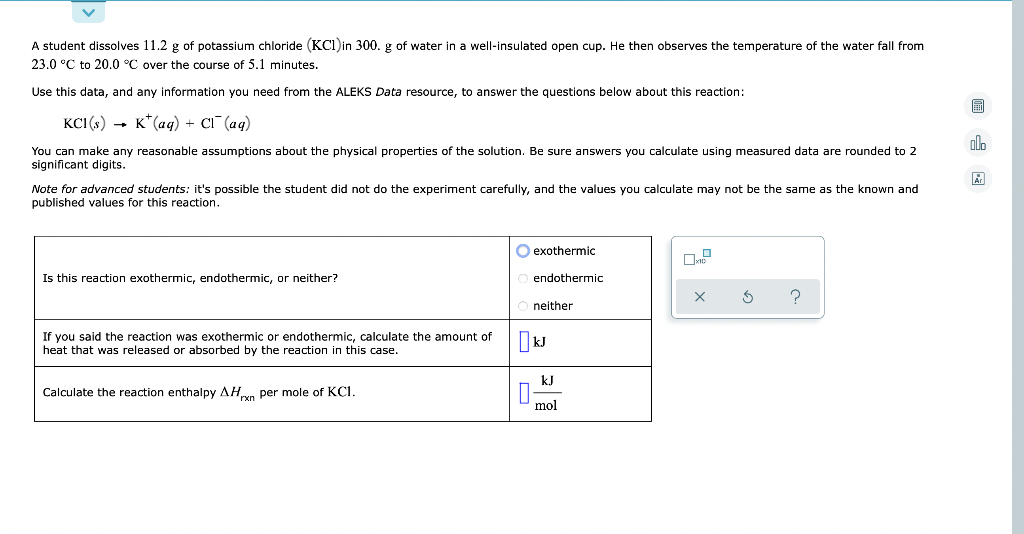
A student dissolves 11.2 g of potassium chloride (KCl) in 300. g of water in a well-insulated open cup. He then observes the temperature of the water fall from 23.0 °C to 20.0 °C over the course of 5.1 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: KCI(s) K+ (aq) + Cl(aq) alo You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to 2 significant digits. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. O exothermic Is this reaction exothermic, endothermic, or neither? ? ? If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. Calculate the reaction enthalpy AHxn per mole of KC1. endothermic neither kJ kJ mol