Home /
Expert Answers /
Chemistry /
a-student-determines-the-area-of-a-rectangle-by-measuring-the-lengths-of-its-sides-using-a-ruler-s-pa229
(Solved): A student determines the area of a rectangle by measuring the lengths of its sides, using a ruler s ...
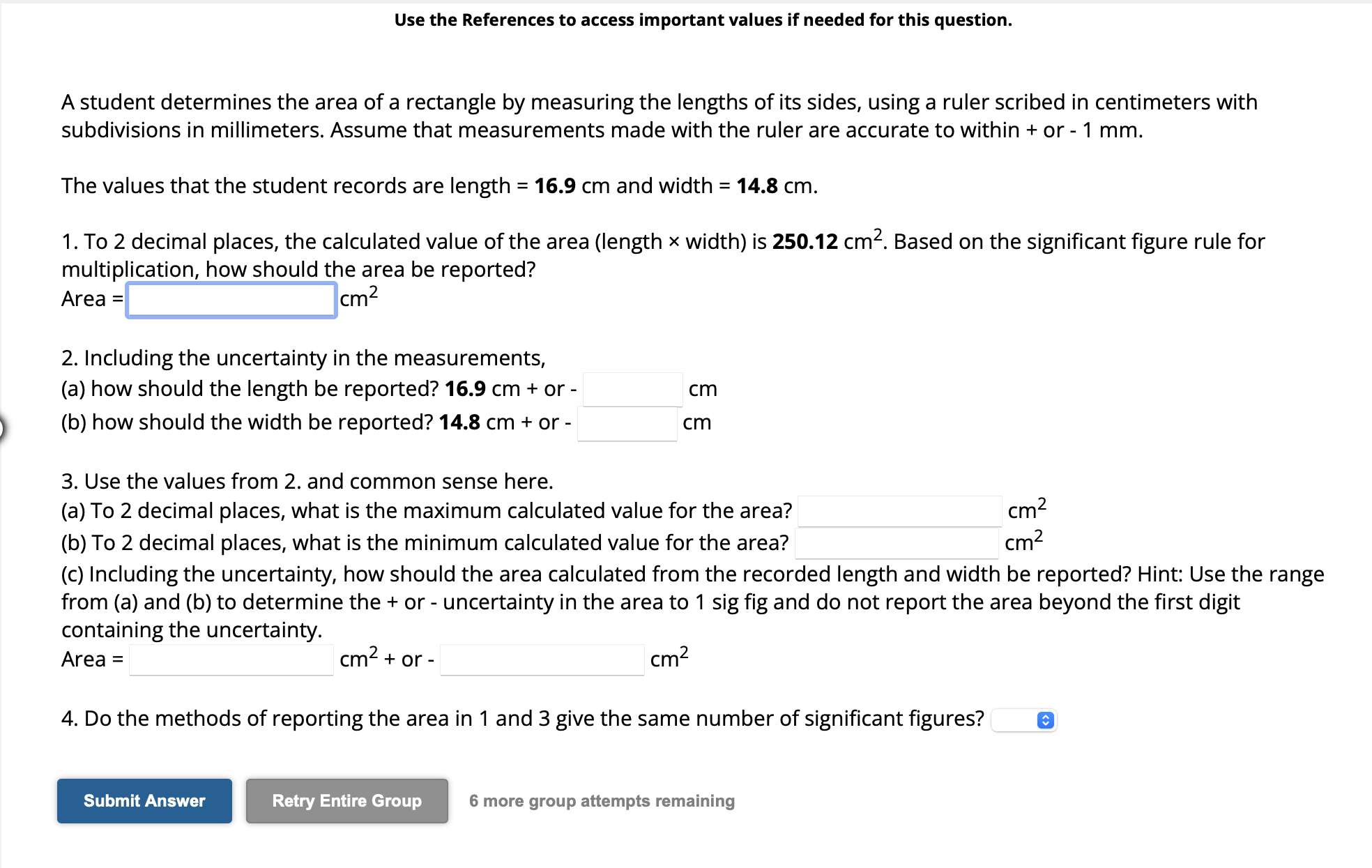
A student determines the area of a rectangle by measuring the lengths of its sides, using a ruler scribed in centimeters with subdivisions in millimeters. Assume that measurements made with the ruler are accurate to within + or . The values that the student records are length and width . 1. To 2 decimal places, the calculated value of the area (length width) is . Based on the significant figure rule for multiplication, how should the area be reported? Area 2. Including the uncertainty in the measurements, (a) how should the length be reported? or - (b) how should the width be reported? or - 3. Use the values from 2. and common sense here. (a) To 2 decimal places, what is the maximum calculated value for the area? (b) To 2 decimal places, what is the minimum calculated value for the area? (c) Including the uncertainty, how should the area calculated from the recorded length and width be reported? Hint: Use the range from (a) and (b) to determine the + or - uncertainty in the area to 1 sig fig and do not report the area beyond the first digit containing the uncertainty. Area or - 4. Do the methods of reporting the area in 1 and 3 give the same number of significant figures? 6 more group attempts remaining
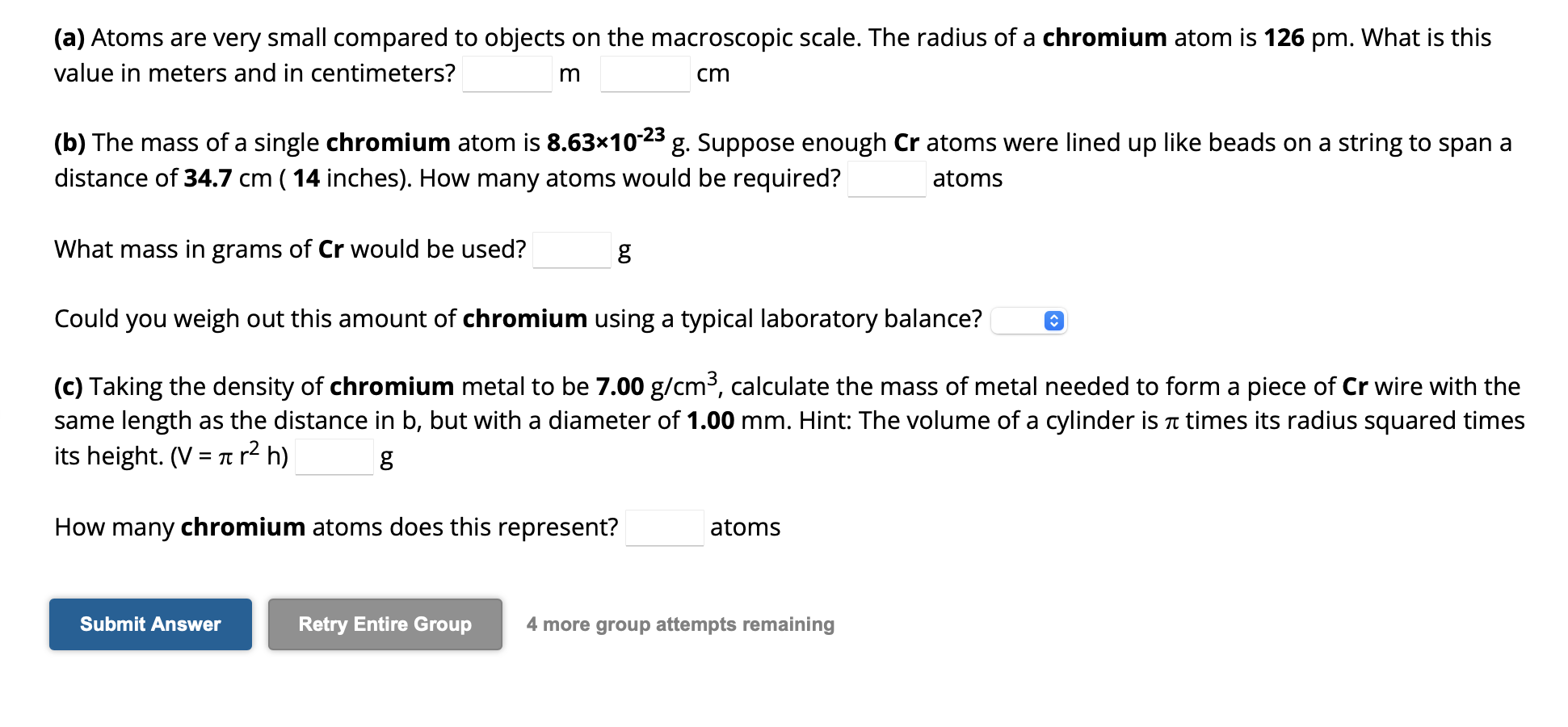
(a) Atoms are very small compared to objects on the macroscopic scale. The radius of a chromium atom is . What is this value in meters and in centimeters? (b) The mass of a single chromium atom is . Suppose enough atoms were lined up like beads on a string to span a distance of ( 14 inches). How many atoms would be required? atoms What mass in grams of would be used? g Could you weigh out this amount of chromium using a typical laboratory balance? (c) Taking the density of chromium metal to be , calculate the mass of metal needed to form a piece of wire with the same length as the distance in , but with a diameter of . Hint: The volume of a cylinder is times its radius squared times its height. How many chromium atoms does this represent? atoms 4 more group attempts remaining